Calmodulin-dependent protein kinase IV regulates nuclear export of Cabin1 during T-cell activation - PubMed (original) (raw)
Calmodulin-dependent protein kinase IV regulates nuclear export of Cabin1 during T-cell activation
Fan Pan et al. EMBO J. 2005.
Abstract
Calcium signaling is critical for activation of T lymphocytes and has been proposed to be transduced through multiple calmodulin target proteins. Whereas the calcineurin-NFAT signaling module is critical for all mammalian T cells, the role of calmodulin-dependent kinase IV (CaMKIV) in mouse naïve CD4+ T-cell activation remains enigmatic. We have applied lentivius-mediated RNA interference of CaMKIV to human T cells and found that knockdown of CaMKIV abrogates T-cell receptor-mediated transcription of the IL-2 gene. We demonstrate that CaMKIV directly phosphorylates Cabin1, a transcriptional corepressor for myocyte enhancer factor 2, creating a docking site for 14-3-3, which causes its nuclear export. CaMKIV-mediated nuclear export of Cabin1 is likely to account for a significant part of the requirement of CaMKIV during human T-cell activation.
Figures
Figure 1
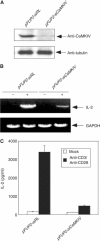
RNA interference of CaMKIV expression leads to inhibition of IL-2 promoter activation. (A) Specific inhibition of CaMKIV expression by CaMKIV siRNA. Lysates prepared from primary human CD4+ naïve T cells transduced with viruses harboring either control pFUP2-RL vector or pFUP2-siCaMKIV were subjected to Western blot analysis using anti-CaMKIV (top panel) or anti-tubulin (bottom panel) antibodies. (B) RNA interference with CaMKIV expression inhibited IL-2 mRNA synthesis in response to stimulation by anti-CD3 and anti-CD28. The two populations of primary human CD4+ naïve T cells were stimulated with a combination of anti-CD3 and anti-CD28 antibodies. Total RNA was prepared from each sample and subjected to RT–PCR analysis. (C) RNA interference with CaMKIV expression inhibited IL-2 secretion in response to stimulation by anti-CD3 plus anti-CD28 antibodies. IL-2 protein secreted into the culture medium was determined by ELISA.
Figure 2
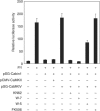
Activated CaMKIV reverses Cabin1-mediated repression of MEF2 transcriptional activity. DO11.10 cells were transfected with MEF2 luciferase reporter plasmid (5 μg) along with plasmids (10 μg each) expressing various proteins as indicated. Transfected cells were allowed to recover for 12 h before they were treated with KN62 (10 μM), W7 (25 μM), W5 (50 μM) or FK506 (1 nM) for 30 min, followed by PMA (40 nM) and ionomycin (1 μM) treatment for another 8 h before cells were lysed for determination of luciferase and β-galactosidase activity.
Figure 3
Ca2+/CaM and CaMKIV synergistically release Cabin1 from MEF2D upon calcium signaling. (A) Myc-tagged full-length Cabin1 plasmid alone or with Flag-tagged constitutively active CaMKIVΔC was transfected into DO11.10 cells. The cell lysates were incubated with _in vitro_-transcribed and translated [35S]MEF2D in the presence of either 2 mM CaCl2 or 10 mM EGTA for 2 h. After immunoprecipitation of Cabin1 with anti-Myc antibody, the bound [35S]MEF2D was visualized by autoradiography. The relative intensity of bound MEF2D was quantitated by PhosphoImage analysis. (B) Co-immunoprecipitation of endogenous MEF2D and Cabin1. Lysates were prepared from DO11.10 cells with or without expression of constitutively active CaMKIVΔC or catalytically inactive inCaMKIV and incubated with anti-MEF2D antibody. And 10 μM KN62 or 25 μM W7 was included in the lysis buffer for cells treated with the same concentrations of each inhibitor. The immunoprecipitates were subject to SDS–PAGE followed by Western blot analysis with anti-Cabin1 antibodies.
Figure 4
Cabin1 is phosphorylated by CaMKIV in vivo and in vitro. (A) Ionomycin treatment increases Cabin1 phosphorylation. Three cell populations, including (1) DO11.10 cells, (2) DO11.10 CaMKIV knockdown (siCaMKIV) and (3) DO11.10 CaMKIV knockdown cells transfected with 10 μg exogenous wild-type CaMKIV, were cultured in media containing 32P for 3 h, followed by either mock or FK506 (1 nM) or KN62 (10 μM) treatment for 30 min prior to treatment with 40 nM PMA and/or 1 μM ionomycin for another 3 h. The cells were lysed, and Cabin1 was immunoprecipitated from each cell extract and analyzed by SDS–PAGE and autoradiography. The total amount of Cabin1 present in each immunoprecipitate was determined by immunoblotting with the Cabin1-specific polyclonal antibodies. (B) In vitro kinase assay. DO11.10 cells were transfected with empty vector alone or with constructs (10 μg each) encoding either human Flag-tagged wild-type CaMKIV or catalytically inactive kinase mutant Flag-CaMKIV/K75E. After a 24 h incubation, cells were treated with 1 μM ionomycin for another 5 min, following which recombinant kinase proteins were immunoprecipitated and incubated with GST-Cabin1-154 (3 μg) in the presence of Ca2+, calmodulin and [γ-32P]ATP.
Figure 5
Identification and verification of CaMKIV phosphorylation site in Cabin1-154. Constitutively active CaMKIV or CaMKII or the corresponding catalytically inactive mutants CaMKIV/K75E or CaMKII/K75E were prepared by using the same protocol described in Figure 4B. Recombinant GST-Cabin154 and GST-Cabin154/S2126A were purified from Escherichia coli lysates by using glutathione-Sepharose beads. The in vitro kinase assay was carried out using the same procedure as described in Figure 4 legend. The GST-Cabin154 or GST-Cabin154/S2126A proteins in each lane were visualized by Coomassie blue staining.
Figure 6
Phosphorylated Cabin1 interacts with 14-3-3. DO11.10 cells were transfected with Myc-tagged Cabin1 plasmid (10 μg) alone or together with CaMKIV expression plasmid (10 μg). At 30 h post-transfection, cells were treated with either mock or 10 μM KN62 for 30 min prior to treatment with 1 μM ionomycin for an additional 8 h. The cells were harvested, lysed and immunoprecipitated with anti-Myc antibody. Cabin1-containing immunoprecipitates were resolved on a 5% SDS–polyacrylamide gel and immunoblotted with anti-Cabin1 antibodies (lower panel) or with 14-3-3τ antibodies (upper panel).
Figure 7
CaM and 14-3-3 bind to Cabin1 simultaneously upon calcium signaling. DO11.10 cells were transiently transfected with 10 μg of pSG-Myc-Cabin1 plasmid alone or along with 10 μg pSG-CaMKIVΔC. After recovery for 24 h, cells were treated with either mock or 10 μM KN62 for 30 min before they were harvested and lysates were prepared. One-tenth of the total cell lysates in each group was set aside as loading control (bottom panel, detected by anti-Myc antibody). The remaining lysates were incubated with either CaM-Sepharose or Sepharose 4B control beads in the presence of either 10 mM EGTA or 2 mM CaCl2 for 2 h. Precipitates were subjected to SDS–PAGE, followed by Western blot analysis using anti-Cabin1 and anti-14-3-3 antibodies.
Figure 8
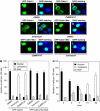
Nuclear export of Cabin1 in response to calcium signaling. Jurkat T cells were transiently transfected with plasmids (10 μg each) encoding GFP-Cabin1 or GFP-Cabin1/S2126A or together with CaMKIVΔC. At 24 h after transfection, cells were treated with DMSO or ionomycin (1 μM) for an additional 6 h, followed by visualization of green GFP (A, C, E, G, I, K) or merged green GFP and blue DAPI staining (B, D, F, H, J, L). (A, B) GFP-Cabin1 is predominantly localized in the nucleus of untreated Jurkat T cells. (C, D) Constitutively active CaMKIV promotes Cabin1 nuclear export. (E, F) Ionomycin treatment causes translocation of GFP-Cabin1 from the nucleus to the cytosol. (G–L) GFP-Cabin1/S2126A following similar treatments as described for wild-type GFP-Cabin1 in panels A–F. GFP-Cabin1/S2126A remains in the nucleus with either CaMKIVΔC coexpression (I, J) or ionomycin treatment (K, L). (M) Quantification of subcellular localization of GFP-Cabin1 and GFP-Cabin1/S2126A mutant. A total of 100 cells were counted for the subcellular distribution of the GFP fusion proteins. (N) Time course of nuclear export of GFP-Cabin1 in response to stimulation by ionomycin in Jurkat T cells.
Figure 9
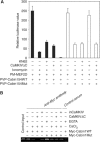
Calmodulin and CaMKIV synergistically dissociate Cabin1 from MEF2 in vivo. (A) Effects of calcium and CaMKIV on the interaction between wild-type or S2126A mutant Cabin1 and MEF2 in a mammalian two-hybrid assay. DO11.10 cells were transfected with 5 μg Gal4-luciferase reporter plasmid along with plasmids (5 μg each) expressing various proteins as indicated. After an overnight recovery, cells were treated with 10 μM KN62 for 30 min prior to treatment with 1 μM ionomycin for an additional 8 h. Cells were harvested and lysed for measurement of luciferase and β-galactosidase activities. (B) Effects of calcium and CaMKIV on association of Cabin1 with MEF2 bound to the endogenous IL-2 promoter. Jurkat T cells were transfected with plasmids (10 μg each) expressing either wild-type or mutant Cabin1 with an N-terminal Myc tag together with plasmids (10 μg each) encoding either constitutively active CaMKIV or an inactive CaMKIV mutant. The transfected cells were incubated at 37°C for 24 h before they were stimulated with ionomycin (1 μM), where indicated, for another 6 h. The cells were then subject to the CHIP assay as described previously with the same PCR primers for DNA fragment containing the MEF2-binding site on the IL-2 promoter (Pan et al, 2004).
Similar articles
- Apoptosis of T cells mediated by Ca2+-induced release of the transcription factor MEF2.
Youn HD, Sun L, Prywes R, Liu JO. Youn HD, et al. Science. 1999 Oct 22;286(5440):790-3. doi: 10.1126/science.286.5440.790. Science. 1999. PMID: 10531067 - Activation protein 1-dependent transcriptional activation of interleukin 2 gene by Ca2+/calmodulin kinase type IV/Gr.
Ho N, Gullberg M, Chatila T. Ho N, et al. J Exp Med. 1996 Jul 1;184(1):101-12. doi: 10.1084/jem.184.1.101. J Exp Med. 1996. PMID: 8691123 Free PMC article. - Regulation and properties of the rat Ca2+/calmodulin-dependent protein kinase IV gene and its protein products.
Means AR, Ribar TJ, Kane CD, Hook SS, Anderson KA. Means AR, et al. Recent Prog Horm Res. 1997;52:389-406; discussion 406-7. Recent Prog Horm Res. 1997. PMID: 9238860 Review. - Ca2+/calmodulin-dependent protein kinase IV and calcium signaling.
Anderson KA, Kane CD. Anderson KA, et al. Biometals. 1998 Dec;11(4):331-43. doi: 10.1023/a:1009276932076. Biometals. 1998. PMID: 10191497 Review.
Cited by
- MEF2 transcription factors: developmental regulators and emerging cancer genes.
Pon JR, Marra MA. Pon JR, et al. Oncotarget. 2016 Jan 19;7(3):2297-312. doi: 10.18632/oncotarget.6223. Oncotarget. 2016. PMID: 26506234 Free PMC article. Review. - Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1.
Dang EV, Barbi J, Yang HY, Jinasena D, Yu H, Zheng Y, Bordman Z, Fu J, Kim Y, Yen HR, Luo W, Zeller K, Shimoda L, Topalian SL, Semenza GL, Dang CV, Pardoll DM, Pan F. Dang EV, et al. Cell. 2011 Sep 2;146(5):772-84. doi: 10.1016/j.cell.2011.07.033. Epub 2011 Aug 25. Cell. 2011. PMID: 21871655 Free PMC article. - Current Insights and Future Prospects for Targeting IL-17 to Treat Patients With Systemic Lupus Erythematosus.
Koga T, Ichinose K, Kawakami A, Tsokos GC. Koga T, et al. Front Immunol. 2021 Feb 1;11:624971. doi: 10.3389/fimmu.2020.624971. eCollection 2020. Front Immunol. 2021. PMID: 33597953 Free PMC article. Review. - Histone deacetylase 3 interacts with and deacetylates myocyte enhancer factor 2.
Grégoire S, Xiao L, Nie J, Zhang X, Xu M, Li J, Wong J, Seto E, Yang XJ. Grégoire S, et al. Mol Cell Biol. 2007 Feb;27(4):1280-95. doi: 10.1128/MCB.00882-06. Epub 2006 Dec 11. Mol Cell Biol. 2007. PMID: 17158926 Free PMC article.
References
- Anderson KA, Ribar TJ, Illario, M, Means AR (1997) Defective survival and activation of thymocytes in transgenic mice expressing a catalytically inactive form of Ca2+/calmodulin-dependent protein kinase IV. Mol Endocrinol 11: 725–737 - PubMed
- Berger I, Bieniossek C, Schaffitzel C, Hassler M, Santelli E, Richmond TJ (2003) Direct interaction of Ca2+/calmodulin inhibits histone deacetylase 5 repressor core binding to myocyte enhancer factor 2. J Biol Chem 278: 17625–17635 - PubMed
- Blaeser F, Ho N, Prywes R, Chatila TA (2000) Ca2+-dependent gene expression mediated by MEF2 transcription factors. J Biol Chem 275: 197–209 - PubMed
- Chatila T, Anderson KA, Ho N, Means AR (1996) A unique phosphorylation-dependent mechanism for the activation of Ca2+/calmodulin-dependent protein kinase type IV/GR. J Biol Chem 271: 21542–21548 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials