Binding of activated alpha2-macroglobulin to its cell surface receptor GRP78 in 1-LN prostate cancer cells regulates PAK-2-dependent activation of LIMK - PubMed (original) (raw)
Binding of activated alpha2-macroglobulin to its cell surface receptor GRP78 in 1-LN prostate cancer cells regulates PAK-2-dependent activation of LIMK
Uma Kant Misra et al. J Biol Chem. 2005.
Abstract
Two characteristics of highly malignant cells are their increased motility and secretion of proteinases allowing these cells to penetrate surrounding basement membranes and metastasize. Activation of 21-kDa activated kinases (PAKs) is an important mechanism for increasing cell motility. Recently, we reported that binding of receptor-recognized forms of the proteinase inhibitor alpha2-macroglobulin (alpha2M*) to GRP78 on the cell surface of 1-LN human prostate cancer cells induces mitogenic signaling and cellular proliferation. In the current study, we have examined the ability of alpha2M* to activate PAK-1 and PAK-2. Exposure of 1-LN cells to alpha2M* caused a 2- to 3-fold increase in phosphorylated PAK-2 and a similar increase in its kinase activity toward myelin basic protein. By contrast, the phosphorylation of PAK-1 was only negligibly affected. Silencing the expression of the GRP78 gene, using either of two different mRNA sequences, greatly attenuated the appearance of phosphorylated PAK-2 in alpha2M*-stimulated cells. Treatment of 1-LN cells with alpha2M* caused translocation of PAK-2 in association with NCK to the cell surface as evidenced by the co-immunoprecipitation of PAK-2 and NCK in the GRP78 immunoprecipitate from plasma membranes. alpha2M*-induced activation of PAK-2 was inhibited by prior incubation of the cells with specific inhibitors of tyrosine kinases and phosphatidylinositol 3-kinase. PAK-2 activation was accompanied by significant increases in the levels of phosphorylated LIMK and phosphorylated cofilin. Silencing the expression of the PAK-2 gene greatly attenuated the phosphorylation of LIMK. In conclusion, we show for the first time the activation of PAK-2 in 1-LN prostate cancer cells by a proteinase inhibitor, alpha2-macroglobulin. These studies suggest a mechanism by which alpha2M* enhances the metastatic potential of these cells.
Figures
Figure 1
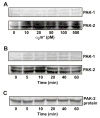
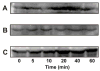
Effect of α2M* on phosphorylation of PAK-2 in 1-LN cells. Western blotting was performed as described under EXPERIMENTAL PROCEDURES. Panel A: Effect of α2M* concentration on the levels of phosphorylated PAK-1 and PAK-2. Panel B: Effect of time of incubation with α2M* on the levels of phosphorylated PAK-1 and phosphorylated PAK-2. Panel C: The protein loading control for PAK-2 is shown. Not shown is the actin loading control. The immunoblots shown here are representative of four to five independent experiments.
Figure 2
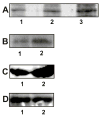
Autophosphorylation and kinase activity of PAKs in 1-LN cells stimulated with α2M*. See EXPERIMENTAL PROCEDURES section for details. Panel A: Autoradiograph showing autophosphorylation of PAK-1 and PAK-2 in the respective immunoprecipitates. The lanes are: (1) buffer control; (2) PAK-1 immunoprecipitate; and lane (3) PAK-2 immunoprecipitate. Panel B: Autoradiograph showing PAK-2 kinase activity towards MBP. The lanes are: (1) buffer control and (2) PAK-2 immunoprecipitate. Panel C: Rac-1·GTP levels in 1-LN-cells treated with (1) buffer and (2) α2M* (50 pM)/10 min); Panel D: Levels of total Rac-1 protein in 1-LN cells treated with (1) buffer and (2) α2M* (50 pM/10 min). Autoradiographs shown are representative of three independent experiments. The immunoblots shown are representative of two individual experiments performed in duplicate.
Figure 3
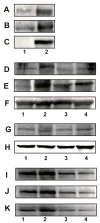
Plasma membrane association of GRP78, NCK, and PAK-2 in 1-LN cells stimulated with α2M*. See EXPERIMENTAL PROCEDURES section for details. Panel A: GRP78 in the GRP78 immunoprecipitate from plasma membrane. Panel B: PAK-2 in the GRP78 immunoprecipitate from plasma membrane. Panel C: NCK in GRP78 immunoprecipitate from plasma membrane. The lanes are: (1) buffer and (2) α2M* (50 pM/10 min). Panel D amd J: Effect of silencing the expression of the GRP78 gene on association of PAK-2. Panel E and K: Effect of silencing the expression of the GRP78 gene on the association of NCK. Panel F: Protein loading control, actin. Panel G and I: Effect of silencing the expression of the GRP78 gene on GRP78 protein levels, and Panel H protein loading control GADPH. The lanes in Panel D, E, F, and G are: (1) buffer; (2) α2M*; (3) dsRNA GRP78 then α2M*; and (4) scrambled dsRNA then α2M*. The lanes in Panel I, J, and K are: (1) buffer; (2) α2M*; (3) dsRNA GRP78 and (4) dsRNA GRP78 then α2M*. The immunoblots shown are representative of three to four independent experiments.
Figure 4
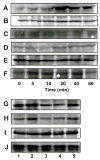
α2M* and the phosphorylation of LIMK and cofilin in 1-LN cells. Panel A: Effect of time of incubation on phosphorylation of LIMK. Panel B: Effect of time of incubation on LIMK protein. Panel C: Protein loading control actin; Panel D: Effect of time of incubation on phosphorylation of cofilin; Panel E: Effect of time of incubation on cofilin protein levels; Panel F: Protein loading control, actin; Panel G: Effect of silencing the expression of the PAK-2 gene on PAK-2 protein levels; Panel H: Effect of silencing the expression of the PAK-2 gene on phosphorylated LIMK; Panel I: Effect of silencing the expression of the PAK-2 gene on LIMK protein levels and Panel J: protein loading control GADPH. Immunoblots shown are representative of three to four independent experiments.
Figure 5
Phosphorylation of Bad in α2M*-stimulated 1-LN cells. Panel A: Effect of time of incubation of 1-LN cells with α2M* (50 pM) on phosphorylation of BAD at Ser112 and Panel B: Effect of time of incubation of 1-LN cells with α2M* (50 pM) on phosphorylation of BAD at Ser136. Panel C: Bad protein. The immunoblots are representative of three to four independent experiments.
Figure 6
Modulation of PAK-2 activity by tyrosine kinase and PI 3-kinase in 1-LN cells stimulated with α2M*. Panel A: Autoradiograph showing MBP phosphorylation by PAK-2. The lanes are: (1) buffer; (2) PAK-2 immunoprecipitate; (3) PAK-2 immunoprecipitate from 1-LN cells treated with genestin (20 μM/16h) before α2M* (50 pM/10 min) stimulation; and (4) PAK-2 immunoprecipitate from 1-LN cells treated with LY294002 (20 μM/20 min) before α2M* stimulation. Panel B: Immunoblot showing the effect of tyrosine kinase and PI 3-kinase inhibitors on levels of phosphorylated PAK-2. The lanes are: (1) buffer; (2) α2M (50 pM/10 min); (3) genestin (20μM/16 h), then α2M* (50 pM/10 min); (4) LY294002 (20 μM/20 min) then α2M*; (5) wortmannin (30 nM/30 min) then α2M*; and (6) LY353511 (20 μM/20 min) then α2M*. The protein loading controls immunoblots of actin are shown below the immunoblot which is representative of three independent experiments.
Figure 7
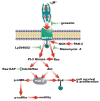
A schematic representation of the mechanisms of PAK-2 activation in 1-LN prostate cancer stimulated with α2M*.
Similar articles
- PAK signaling in oncogenesis.
Molli PR, Li DQ, Murray BW, Rayala SK, Kumar R. Molli PR, et al. Oncogene. 2009 Jul 16;28(28):2545-55. doi: 10.1038/onc.2009.119. Epub 2009 May 25. Oncogene. 2009. PMID: 19465939 Free PMC article. Review. - Activation and cross-talk between Akt, NF-kappaB, and unfolded protein response signaling in 1-LN prostate cancer cells consequent to ligation of cell surface-associated GRP78.
Misra UK, Deedwania R, Pizzo SV. Misra UK, et al. J Biol Chem. 2006 May 12;281(19):13694-13707. doi: 10.1074/jbc.M511694200. Epub 2006 Mar 16. J Biol Chem. 2006. PMID: 16543232 - Activated α2-macroglobulin binding to cell surface GRP78 induces T-loop phosphorylation of Akt1 by PDK1 in association with Raptor.
Misra UK, Pizzo SV. Misra UK, et al. PLoS One. 2014 Feb 6;9(2):e88373. doi: 10.1371/journal.pone.0088373. eCollection 2014. PLoS One. 2014. PMID: 24516643 Free PMC article. Retracted. - Regulation of phosphorylation pathways by p21 GTPases. The p21 Ras-related Rho subfamily and its role in phosphorylation signalling pathways.
Lim L, Manser E, Leung T, Hall C. Lim L, et al. Eur J Biochem. 1996 Dec 1;242(2):171-85. doi: 10.1111/j.1432-1033.1996.0171r.x. Eur J Biochem. 1996. PMID: 8973630 Review.
Cited by
- FOXM1 promotes invasion and migration of colorectal cancer cells partially dependent on HSPA5 transactivation.
Luo X, Yao J, Nie P, Yang Z, Feng H, Chen P, Shi X, Zou Z. Luo X, et al. Oncotarget. 2016 May 3;7(18):26480-95. doi: 10.18632/oncotarget.8419. Oncotarget. 2016. PMID: 27034162 Free PMC article. - Association between glucose-regulated protein and neutrophil apoptosis in severe acute pancreatitis.
Xu LT, Xu HL, Fu MS. Xu LT, et al. Int J Clin Exp Pathol. 2015 Aug 1;8(8):9300-6. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26464680 Free PMC article. - The cell surface GRP78 facilitates the invasion of hepatocellular carcinoma cells.
Zhang XX, Li HD, Zhao S, Zhao L, Song HJ, Wang G, Guo QJ, Luan ZD, Su RJ. Zhang XX, et al. Biomed Res Int. 2013;2013:917296. doi: 10.1155/2013/917296. Epub 2013 Dec 8. Biomed Res Int. 2013. PMID: 24383061 Free PMC article. Retracted. - PAK signaling in oncogenesis.
Molli PR, Li DQ, Murray BW, Rayala SK, Kumar R. Molli PR, et al. Oncogene. 2009 Jul 16;28(28):2545-55. doi: 10.1038/onc.2009.119. Epub 2009 May 25. Oncogene. 2009. PMID: 19465939 Free PMC article. Review. - Retraction: Receptor-Recognized α2-Macroglobulin Binds to Cell Surface-Associated GRP78 and Activates mTORC1 and mTORC2 Signaling in Prostate Cancer Cells.
PLOS One Editors. PLOS One Editors. PLoS One. 2025 Jun 5;20(6):e0325675. doi: 10.1371/journal.pone.0325675. eCollection 2025. PLoS One. 2025. PMID: 40471893 Free PMC article. No abstract available.
References
- Jemal A, Thomas A, Murray T, Thun M. CA Cancer J Clin. 2002;52:23–47. - PubMed
- Heinlein CA, Chang C. Endocr Rev. 2004;25:276–308. - PubMed
- Montano X, Djamgoz M. FEBS Lett. 2004;571:1–8. - PubMed
- Schlessinger J. Cell. 2000;103:193–200. - PubMed
- Sells MA, Chernoff J. Trends Cell Biol. 1997;7:162–167. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous