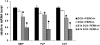Endoplasmic reticulum stress modulates the response of myelinating oligodendrocytes to the immune cytokine interferon-gamma - PubMed (original) (raw)
Endoplasmic reticulum stress modulates the response of myelinating oligodendrocytes to the immune cytokine interferon-gamma
Wensheng Lin et al. J Cell Biol. 2005.
Abstract
Interferon-gamma (IFN-gamma) is believed to contribute to immune-mediated demyelinating disorders by targeting the myelin-producing oligodendrocyte, a cell known to be highly sensitive to the disruption of protein synthesis and to the perturbation of the secretory pathway. We found that apoptosis induced by IFN-gamma in cultured rat oligodendrocytes was associated with endoplasmic reticulum (ER) stress. ER stress also accompanied oligodendrocyte apoptosis and hypomyelination in transgenic mice that inappropriately expressed IFN-gamma in the central nervous system (CNS). Compared with a wild-type genetic background, the enforced expression of IFN-gamma in mice that were heterozygous for a loss of function mutation in pancreatic ER kinase (PERK) dramatically reduced animal survival, promoted CNS hypomyelination, and enhanced oligodendrocyte loss. PERK encodes an ER stress-inducible kinase that phosphorylates eukaryotic translation initiation factor 2alpha and specifically maintains client protein homeostasis in the stressed ER. Therefore, the hypersensitivity of PERK+/- mice to IFN-gamma implicates ER stress in demyelinating disorders that are induced by CNS inflammation.
Figures
Figure 1.
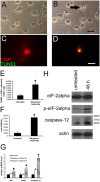
IFN-γ–induced apoptosis in cultured rat oligodendrocytes is associated with ER stress. (A) Untreated oligodendrocytes that underwent differentiation for 7 d. (B) Oligodendrocytes that underwent differentiation for 5 d and treatment with 70 U/ml IFN-γ for 48 h, revealing cell shrinkage and aggregation of cell bodies (arrow). (C and D) TUNEL and CNP double labeling for untreated oligodendrocytes that underwent differentiation for 7 d (C) and for oligodendrocytes that underwent differentiation for 5 d and treatment with 70 U/ml IFN-γ for 48 h (D). (E) Quantitation of TUNEL and CNPase double positive cells; *, P < 0.05. (F) Caspase-3 activity assay in the oligodendrocyte lysates; *, P < 0.01. (G) Real-time PCR analyses of the expression of BIP, CHOP, and caspase-12 in oligodendrocytes treated with 70 U/ml IFN-γ; *, P < 0.05. (E–G) Error bars represent standard deviation. (H) Western blot analyses of total eIF-2α, p-eIF-2α, and caspase-12 in oligodendrocytes treated with 70 U/ml IFN-γ. All experiments were repeated at least three times. Bars: (A and B) 30 μM; (C and D) 20 μM.
Figure 2.
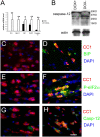
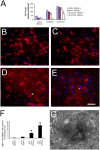
Hypomyelination induced by ectopically expressed IFN-γ is associated with ER stress. (A) Real-time PCR analyses for detection of mRNA in the brains of 14-d-old mice ectopically expressing IFN-γ (n = 3); *, P < 0.05; **, P < 0.01. Error bars represent standard deviation. (B) Western blot analyses for caspase-12 in the CNS of 14-d-old double transgenic mice released from doxyclycline at E 14. (C and D) BIP and CC1 double immunostaining in the spinal cord of 14-d-old double transgenic mice that received doxycycline (C) or were released from doxycycline at E 14 (D). (E and F) p-eIF-2α and CC1 double immunostaining in the spinal cord of 14-d-old double transgenic mice that received doxycycline (E) or were released from doxycycline at E 14 (F). (G and H) Caspase-12 and CC1 double immunostaining in the spinal cord of 14-d-old double transgenic mice that received doxycycline (G) or were released from doxycycline at E 14 (H). (C–H) n = 3; bar, 30 μM.
Figure 3.
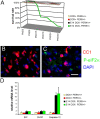
Hypersensitivity of PERK+_/_− mice to the conditional misexpression of IFN-γ. (A) Mouse survival curve (n = 40 for each group). (B and C) p-eIF-2α and CC1 double labeling in the spinal cord of 14-d-old GFAP/tTA; TRE/IFN-γ; PERK+_/_− mice that received doxycycline (B) or were released from doxycycline at E 14 (C). (B and C) n = 3; bar, 30 μM. (D) Real-time PCR analyses of mRNA levels in the brain of 14-d-old mice (n = 3). Error bars represent standard deviation.
Figure 4.
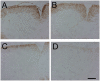
Double transgenic mice with a PERK+_/_− background develop severe hypomyelination. (A and C) MBP immunostaining in the spinal cord of 14-d-old double transgenic mice that received doxycycline (A) or were released from doxycycline at E 14 (C). (B and D) MBP immunostaining in the spinal cord of 14-d-old GFAP/tTA; TRE/IFN-γ; PERK+_/_− mice that received doxycycline (B) or were released from doxycycline at E 14 (D). (A–D) n = 3; bar, 150 μM.
Figure 5.
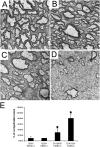
Double transgenic mice with a PERK+_/_− background develop severe hypomyelination. (A and B) Ultrastructural examination showing normal myelination in the spinal cord of 14-d-old double transgenic mice (A) and GFAP/tTA; TRE/IFN-γ; PERK+_/− mice (B) that received doxycycline. (C and D) Ultrastructural examination showing minor hypomyelination in the spinal cord of 14-d-old double transgenic mice (C) and severe hypomyelination in the spinal cord of 14-d-old GFAP/tTA; TRE/IFN-γ; PERK+/_− mice (D) released from doxycycline at E 14. (A–D) n = 3; bars, 1 μM. (E) The percentage of unmyelinated axons in the white matter of the cervical spinal cord was calculated from three mice per time point; *, P < 0.01. Error bars represent standard deviation.
Figure 6.
The levels of MBP, PLP, and CGT mRNA were significantly decreased in the CNS of double transgenic mice with a PERK+_/_− background. Real-time PCR analyses for myelin gene expression in the brain of 14-d-old mice (n = 3); *, P < 0.05. Error bars represent standard deviation.
Figure 7.
Double transgenic mice with a PERK+_/_− background lose the majority of oligodendrocytes in the CNS. (A) Quantitation of CC1-positive cells in the CNS of 14-d-old mice (n = 3); *, P < 0.05. (B and C) TUNEL and CC1 double labeling in the spinal cord of 14-d-old double transgenic mice (B) and GFAP/tTA; TRE/IFN-γ; PERK+_/− mice (C) that received doxycycline. (D and E) TUNEL and CC1 double labeling in the spinal cord of 14-d-old double transgenic mice (D) and GFAP/tTA; TRE/IFN-γ; PERK+/_− mice (E) released from doxycycline at E 14. (B–E) n = 3; bar, 60 μM; red fluorescence shows CC1 immunoreactivity; green fluorescence shows TUNEL stain; and blue fluorescence shows DAPI countstain. (F) Quantitation of TUNEL and CC1 double positive cells in the spinal cord of 14-d-old mice (n = 3); *, P < 0.01. (A and F) Error bars represent standard deviation. (G) Ultrastructural examination showing that apoptotic oligodendrocytes contained highly condensed chromatin mass, intact membrane, shrunken cytoplasm, and apoptosis body; bar, 2 μM.
Figure 8.
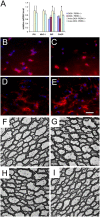
Oligodendrocytes in adult mice are less sensitive to IFN-γ than actively myelinating oligodendrocytes from younger mice. (A) Real-time PCR analyses of mRNA levels in the brains of 10-wk-old mice (n = 3); *, P < 0.05. Error bars represent standard deviation. (B and C) BIP and CC1 double immunostaining in the cerebellum of 10-wk-old double transgenic mice (B) and GFAP/tTA; TRE/IFN-γ; PERK+_/− mice (C) that received doxycycline. (D and E) BIP and CC1 double immunostaining in the cerebellum of 10-wk-old double transgenic mice (D) and GFAP/tTA; TRE/IFN-γ; PERK+/− mice (E) released from doxycycline at 4 wk of age. (B–E) n = 3; bar, 60 μM; red fluorescence shows CC1 immunoreactivity; absence of green fluorescence shows that no cells express detectable levels of BIP; and blue fluorescence shows DAPI countstain. (F and G) Ultrastructural examination showing normal myelination in the cerebellum of 10-wk-old double transgenic mice (F) and GFAP/tTA; TRE/IFN-γ; PERK+/− mice (G) that received doxycycline. (H and I) Ultrastructural examination showing normal myelination in the cerebellum of 10-wk-old double transgenic mice (H) and GFAP/tTA; TRE/IFN-γ; PERK+/_− mice (I) released from doxycycline at 4 wk of age. (F–I) n = 3; bars, 2 μM.
Similar articles
- Enhanced integrated stress response promotes myelinating oligodendrocyte survival in response to interferon-gamma.
Lin W, Kunkler PE, Harding HP, Ron D, Kraig RP, Popko B. Lin W, et al. Am J Pathol. 2008 Nov;173(5):1508-17. doi: 10.2353/ajpath.2008.080449. Epub 2008 Sep 25. Am J Pathol. 2008. PMID: 18818381 Free PMC article. - Interferon-gamma inhibits central nervous system remyelination through a process modulated by endoplasmic reticulum stress.
Lin W, Kemper A, Dupree JL, Harding HP, Ron D, Popko B. Lin W, et al. Brain. 2006 May;129(Pt 5):1306-18. doi: 10.1093/brain/awl044. Epub 2006 Feb 27. Brain. 2006. PMID: 16504972 - The integrated stress response prevents demyelination by protecting oligodendrocytes against immune-mediated damage.
Lin W, Bailey SL, Ho H, Harding HP, Ron D, Miller SD, Popko B. Lin W, et al. J Clin Invest. 2007 Feb;117(2):448-56. doi: 10.1172/JCI29571. J Clin Invest. 2007. PMID: 17273557 Free PMC article. - Oligodendroglial response to the immune cytokine interferon gamma.
Popko B, Baerwald KD. Popko B, et al. Neurochem Res. 1999 Feb;24(2):331-8. doi: 10.1023/a:1022586726510. Neurochem Res. 1999. PMID: 9972883 Review. - Dysmyelination in class I MHC transgenic mice.
Turnley AM, Morahan G. Turnley AM, et al. Microsc Res Tech. 1995 Nov 1;32(4):286-94. doi: 10.1002/jemt.1070320403. Microsc Res Tech. 1995. PMID: 8573778 Review.
Cited by
- Immune response in peripheral axons delays disease progression in SOD1G93A mice.
Nardo G, Trolese MC, de Vito G, Cecchi R, Riva N, Dina G, Heath PR, Quattrini A, Shaw PJ, Piazza V, Bendotti C. Nardo G, et al. J Neuroinflammation. 2016 Oct 7;13(1):261. doi: 10.1186/s12974-016-0732-2. J Neuroinflammation. 2016. PMID: 27717377 Free PMC article. - A little stress is good: IFN-gamma, demyelination, and multiple sclerosis.
Lees JR, Cross AH. Lees JR, et al. J Clin Invest. 2007 Feb;117(2):297-9. doi: 10.1172/JCI31254. J Clin Invest. 2007. PMID: 17273549 Free PMC article. - ZFP191 is required by oligodendrocytes for CNS myelination.
Howng SY, Avila RL, Emery B, Traka M, Lin W, Watkins T, Cook S, Bronson R, Davisson M, Barres BA, Popko B. Howng SY, et al. Genes Dev. 2010 Feb 1;24(3):301-11. doi: 10.1101/gad.1864510. Epub 2010 Jan 15. Genes Dev. 2010. PMID: 20080941 Free PMC article. - HLA-B27 misfolding and spondyloarthropathies.
Colbert RA, DeLay ML, Layh-Schmitt G, Sowders DP. Colbert RA, et al. Prion. 2009 Jan-Mar;3(1):15-26. doi: 10.4161/pri.3.1.8072. Epub 2009 Jan 3. Prion. 2009. PMID: 19363299 Free PMC article. Review. - Enhanced integrated stress response promotes myelinating oligodendrocyte survival in response to interferon-gamma.
Lin W, Kunkler PE, Harding HP, Ron D, Kraig RP, Popko B. Lin W, et al. Am J Pathol. 2008 Nov;173(5):1508-17. doi: 10.2353/ajpath.2008.080449. Epub 2008 Sep 25. Am J Pathol. 2008. PMID: 18818381 Free PMC article.
References
- Andrews, T., P. Zhang, and N.R. Bhat. 1998. TNFalpha potentiates IFNgamma-induced cell death in oligodendrocyte progenitors. J. Neurosci. Res. 54:574–583. - PubMed
- Baerwald, K.D., and B. Popko. 1998. Developing and mature oligodendrocytes respond differently to the immune cytokine interferon-gamma. J. Neurosci. Res. 52:230–239. - PubMed
- Baerwald, K.D., J.D. Corbin, and B. Popko. 2000. Major histocompatibility complex heavy chain accumulation in the endoplasmic reticulum of oligodendrocytes results in myelin abnormalities. J. Neurosci. Res. 59:160–169. - PubMed
- Baud, O., J. Li, Y. Zhang, R.L. Neve, J.J. Volpe, and P.A. Rosenberg. 2004. Nitric oxide-induced cell death in developing oligodendrocytes is associated with mitochondrial dysfunction and apoptosis-inducing factor translocation. Eur. J. Neurosci. 20:1713–1726. - PubMed
- Bauer, J., M. Bradl, M. Klein, M. Leisser, T.L. Deckwerth, H. Wekerle, and H. Lassmann. 2002. Endoplasmic reticulum stress in PLP-overexpressing transgenic rats: gray matter oligodendrocytes are more vulnerable than white matter oligodendrocytes. J. Neuropathol. Exp. Neurol. 61:12–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK047119/DK/NIDDK NIH HHS/United States
- R01 ES008681/ES/NIEHS NIH HHS/United States
- ESO8681/ES/NIEHS NIH HHS/United States
- R37 DK047119/DK/NIDDK NIH HHS/United States
- R01 NS034939/NS/NINDS NIH HHS/United States
- DK47119/DK/NIDDK NIH HHS/United States
- NS34939/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases