Structurally and functionally unique complexins at retinal ribbon synapses - PubMed (original) (raw)
Structurally and functionally unique complexins at retinal ribbon synapses
Kerstin Reim et al. J Cell Biol. 2005.
Abstract
Ribbon synapses in retinal sensory neurons maintain large pools of readily releasable synaptic vesicles. This allows them to release several hundreds of vesicles per second at every presynaptic release site. The molecular components that cause this high transmitter release efficiency of ribbon synapses are unknown. In the present study, we identified and characterized two novel vertebrate complexins (CPXs), CPXs III and IV, that are the only CPX isoforms present in retinal ribbon synapses. CPXs III and IV are COOH-terminally farnesylated, and, like CPXs I and II, bind to SNAP receptor complexes. CPXs III and IV can functionally replace CPXs I and II, and their COOH-terminal farnesylation regulates their synaptic targeting and modulatory function in transmitter release. The novel CPXs III and IV may contribute to the unique release efficacy of retinal sensory neurons.
Figures
Figure 1.
Comparison of primary structures of different CPXs in different species. (A) Amino acid sequences of CPXs are shown in single letter amino acid code and aligned for maximal homology. Residues that are identical in the majority of sequences are shown on black background, and residues that are similar are shaded (similarity groups: F, Y, W; I, L, V, M; H, R, K; D, E; G, A; T, S; N, Q). The core helix (aa 48–70 of mCPX I) mediating SNARE complex binding of CPX I is marked with a hatched bar. ag, Anopheles gambia; ce, Caenorhabditis elegans; ci, Ciona intestinalis; dm, Drosophila melanogaster; h, human; hm, Hirudo medicinalis; lp, Loligo pealeii; m, mouse; nj, Narke japonica; xl, Xenopus laevis; GenBank/EMBL/DDBJ accession nos. of the novel CPXs are: AY28650, hCPX III; AY286502, hCPX IV; AY264290, mCPX III; AY264291, mCPX IV. (B) Phylogenetic tree illustrating the homology between different CPXs. Abbreviations as in A.
Figure 2.
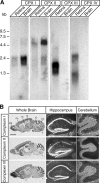
CPX mRNA expression in brain and retina. (A) Blots containing poly(A)+-RNA from rat brain and retina, hybridized at high stringency with uniformly labeled full-length cDNA probes for rCPXs I or II, or mCPXs III or IV, and exposed to film for 20 h. (B) Negative X-ray film images showing the distribution of CPXs I, II, and III in adult mouse brain (left), and high magnification bright field images of emulsion dipped slices where silver grains (bright areas) represent the distribution of CPXs I, II, and III in adult mouse hippocampus (middle) and cerebellum (right). Ce, cerebellum; Co, cortex; Hi, hippocampus; IC, inferior colliculus; OB, olfactory bulb; Th, thalamus. Bar: (left) 2 mm and (middle and right) 0.25 mm.
Figure 3.
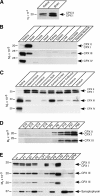
CPX protein expression in different tissues, brain regions, developmental stages, and brain subcellular fractions. Homogenates from the indicated rat organs (A and B), rat brain regions (C), brains from mice of different ages (D), and rat cortex subcellular fractions (E) were analyzed by Western blotting using specific antibodies to the indicated proteins (arrows). The two CPX III panels in E show a short (bottom) and long (top) exposure of the same blot. Developmental stages were designated as follows: E, embryonic day; P, postnatal day. Subcellular fractions were designated as described in Materials and methods.
Figure 4.
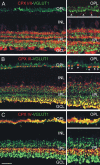
Differential distribution of CPXs I/II, III, and IV in the mouse retina. (A–C) Confocal micrographs of vertical sections through mouse retina double labeled for the different CPXs (red) and VGLUT1 (green), as a marker for the glutamatergic synaptic terminals of photoreceptors and bipolar cells. The micrographs to the right show high power views of areas of the OPL and the IPL. (A) Somata of amacrine cells in the INL and of amacrine and ganglion cells in the GCL show CPX I/II immunoreactivity. The processes of CPX I/II labeled amacrine cells stratify in three bands in the IPL. In the OPL, horizontal cell processes are weakly CPX I/II immunoreactive (arrows). There is no colocalization of CPX I/II and VGLUT1. (B) Somata of amacrine cells in the INL and their processes stratifying in a broad band in the IPL, abutting the INL, are labeled for CPX III. The large CPX III immunoreactive structures in the IPL, close to the GCL, and in the OPL, are bipolar and cone photoreceptor terminals (arrows), respectively, as seen in the double labeling with VGLUT1. (C) CPX IV and VGLUT1 colocalize in the IPL and the OPL, indicating the expression of CPX IV in bipolar and photoreceptor cell terminals. OPL, outer plexiform layer (synaptic); INL, inner nuclear layer; IPL, inner plexiform layer (synaptic); GCL, ganglion cell layer. Bar, 20 μm.
Figure 5.
Differential expression of CPXs III and IV by photoreceptors and their synapses. (A–C) Confocal micrographs of a vertical section through mouse retina double labeled for CPX III (A, red) and PNA (B, green) as a marker for cone photoreceptor terminals. As seen in the merge of the two stainings, CPX III is strongly expressed in the large cone photoreceptor terminals (C, arrows), and only weakly in the small rod photoreceptor terminals. (D–F) Confocal micrographs of a vertical section through mouse retina double labeled for CPX IV (D, red) and PNA (E, green). The terminals of cone photoreceptors are devoid of CPX IV staining, which is present in the rod photoreceptor terminals (F). (G and H) Confocal micrographs of vertical sections through mouse retina double labeled for CPX III (G, red) and Bassoon (G, green) and for CPX IV (H, red) and Bassoon (H, green). The merge of the stainings confirms the presence of CPX III at cone (G, arrows) and rod ribbon synapses, and of CPX IV at rod ribbon synapses (H). The asterisk in H marks an unspecifically stained blood vessel. Bars, 5 μm.
Figure 6.
In vivo farnesylation of CPXs III and IV. Transfected HEK293 cells coexpressing a mevalonate transporter and either wt EGFP-CPXs III or IV (CPX III, CPX IV), or mutant EGFP-CPXs III or IV (CPX III-C156S, CPX IV-C158S) were labeled with [14C]mevalonate and harvested. Control cells were only transfected with the mevalonate transporter. Whole cell lysates as well as solubilized and immunoprecipitated proteins were analyzed by Western blotting, using an mAb to EGFP (Western blot), and autoradiography (Autoradiograph). The arrows indicate the EGFP-CPX–fusion proteins. All proteins were expressed and precipitated (bottom) but only wt proteins were 14C-labeled (top).
Figure 7.
Distribution of CPXs III and IV in neurons. Neurons transfected with expression vectors encoding either wt CPX I-EGFP, CPX II-EGFP, EGFP-CPX III, EGFP-CPX IV or farnesylation-deficient EGFP-CPX III-C156S, and EGFP-CPX IV-C158S (all green), and immunostained for synaptophysin (red) to mark presynaptic terminals were analyzed by laser scanning confocal microscopy. Wt CPXs III (top left) and IV (middle left) were concentrated in presynaptic structures of axons and colocalized with synaptophysin, whereas mutant CPXs III (top right) and IV (middle right) as well as wt CPXs I (bottom left) and II (bottom right) showed a diffuse axonal distribution with minor (CPXs I and II) or no (mutant CPXs III and IV) presynaptic accumulation. Bars: (low magnification, large images) 50 μm; (high magnification, small images) 10 μm.
Figure 8.
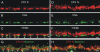
Characteristics of CPXs III and IV function. (A) Cosedimentation assays with CPXs I, III, and IV. CPXs I, III, and IV were expressed as GST-fusion proteins, immobilized on glutathione agarose, and incubated with solubilized proteins from crude rat brain synaptosomes or rat retina homogenate. GST-CPX I and GST alone were used as positive and negative controls, respectively. Bound material was analyzed by Western blotting using antibodies to the indicated proteins. Both, CPXs III and IV bind to the SNARE complex, but the necessity to reduce the stringency of the washing steps in order to demonstrate the interaction between the SNARE complex and CPX IV indicates a lower binding affinity. (B–E) Synaptic amplitudes, RRPs and vesicular release probability in glutamatergic CPX I/II DKO neurons and CPX I/II DKO neurons rescued by overexpression of CPXs I, III, or IV. Representative traces showing autaptic EPSCs and responses to hypertonic sucrose solution from a CPX I/II DKO neuron (B, left), and CPX I/II DKO neurons rescued with wt CPX I (C, right), wt CPX III (C, left), CPX III-C156S (C, right), wt CPX IV (D, left), or CPX IV-C158S (D; right). Horizontal bar EPSC, 0.1 s; sucrose solution, 1.5 s. Vertical bar EPSC, 2 nA; sucrose solution, 1 nA. (E) Bar diagrams summarizing mean vesicular release probability for CPX I/II DKO neurons and CPX I/II DKO neurons rescued with wt CPX I, wt CPX III, CPX III-C156S, wt CPX IV, or CPX IV-C158S. Asterisks indicate P < 0.0001. Error bars indicate SEM.
Similar articles
- Presynaptic proteins of ribbon synapses in the retina.
Morgans CW. Morgans CW. Microsc Res Tech. 2000 Jul 15;50(2):141-50. doi: 10.1002/1097-0029(20000715)50:2<141::AID-JEMT6>3.0.CO;2-B. Microsc Res Tech. 2000. PMID: 10891878 Review. - Differential distribution of vesicle associated membrane protein isoforms in the mouse retina.
Sherry DM, Wang MM, Frishman LJ. Sherry DM, et al. Mol Vis. 2003 Dec 11;9:673-88. Mol Vis. 2003. PMID: 14685145 - Absence of functional active zone protein Bassoon affects assembly and transport of ribbon precursors during early steps of photoreceptor synaptogenesis.
Regus-Leidig H, tom Dieck S, Brandstätter JH. Regus-Leidig H, et al. Eur J Cell Biol. 2010 Jun;89(6):468-75. doi: 10.1016/j.ejcb.2009.12.006. Epub 2010 Feb 25. Eur J Cell Biol. 2010. PMID: 20188438 - Aberrant function and structure of retinal ribbon synapses in the absence of complexin 3 and complexin 4.
Reim K, Regus-Leidig H, Ammermüller J, El-Kordi A, Radyushkin K, Ehrenreich H, Brandstätter JH, Brose N. Reim K, et al. J Cell Sci. 2009 May 1;122(Pt 9):1352-61. doi: 10.1242/jcs.045401. J Cell Sci. 2009. PMID: 19386896 - [Synaphins/complexins, cytosolic proteins associated for neurotransmitter release].
Ishizuka T, Abe T. Ishizuka T, et al. Tanpakushitsu Kakusan Koso. 2000 Feb;45(3 Suppl):449-55. Tanpakushitsu Kakusan Koso. 2000. PMID: 10707655 Review. Japanese. No abstract available.
Cited by
- Molecularly Defined Subplate Neurons Project Both to Thalamocortical Recipient Layers and Thalamus.
Viswanathan S, Sheikh A, Looger LL, Kanold PO. Viswanathan S, et al. Cereb Cortex. 2017 Oct 1;27(10):4759-4768. doi: 10.1093/cercor/bhw271. Cereb Cortex. 2017. PMID: 27655928 Free PMC article. - Effects of glaucoma on Chrna6 expression in the retina.
Munguba GC, Geisert EE, Williams RW, Tapia ML, Lam DK, Bhattacharya SK, Lee RK. Munguba GC, et al. Curr Eye Res. 2013 Jan;38(1):150-7. doi: 10.3109/02713683.2012.724512. Epub 2012 Sep 24. Curr Eye Res. 2013. PMID: 23002780 Free PMC article. - Accessory alpha-helix of complexin I can displace VAMP2 locally in the complexin-SNARE quaternary complex.
Lu B, Song S, Shin YK. Lu B, et al. J Mol Biol. 2010 Feb 26;396(3):602-9. doi: 10.1016/j.jmb.2009.12.020. Epub 2009 Dec 21. J Mol Biol. 2010. PMID: 20026076 Free PMC article. - Synaptic vesicles position complexin to block spontaneous fusion.
Wragg RT, Snead D, Dong Y, Ramlall TF, Menon I, Bai J, Eliezer D, Dittman JS. Wragg RT, et al. Neuron. 2013 Jan 23;77(2):323-34. doi: 10.1016/j.neuron.2012.11.005. Neuron. 2013. PMID: 23352168 Free PMC article.
References
- Blanks, J.C., and L.V. Johnson. 1984. Specific binding of peanut lectin to a class of retinal photoreceptor cells. A species comparison. Invest. Ophthalmol. Vis. Sci. 25:546–557. - PubMed
- Brandstätter, J.H., E.L. Fletcher, C.C. Garner, E.D. Gundelfinger, and H. Wässle. 1999. Differential expression of the presynaptic cytomatrix protein Bassoon among ribbon synapses in the mammalian retina. Eur. J. Neurosci. 11:3683–3693. - PubMed
- Chen, X., D.R. Tomchick, E. Kovrigin, D. Arac, M. Machius, T.C. Südhof, and J. Rizo. 2002. Three-dimensional structure of the complexin/SNARE complex. Neuron. 33:397–409. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases