Spared nerve injury rats exhibit thermal hyperalgesia on an automated operant dynamic thermal escape task - PubMed (original) (raw)
Comparative Study
Spared nerve injury rats exhibit thermal hyperalgesia on an automated operant dynamic thermal escape task
Marwan Baliki et al. Mol Pain. 2005.
Abstract
Well-established methods are available to measure thermal and mechanical sensitivity in awake behaving rats. However, they require experimenter manipulations and tend to emphasize reflexive behaviors. Here we introduce a new behavioral test, with which we examine thermal sensitivity of rats with neuropathic injury. We contrast thermal hyperalgesia between spared nerve injury and chronic constriction injury rats. This device is a fully automated thermal sensitivity assessment tool designed to emphasize integrated learned responses to thermal painful and non-painful stimuli that are applied dynamically to a surface on which the animal is standing. It documents escape behavior in awake, unrestrained animals to innocuous and noxious heating of the floor where the animal is located. Animals learn to minimize pain by escaping to the opposite non-heated side; escape latency is recorded. On this device, thermal stimulus-response curves showed > 6 degrees C leftward shift in both groups of neuropathic rats. In contrast, when these animals were tested on hotplate the stimulus-response shift was < 2 degrees C. Spared nerve injury rats showed even less evidence for thermal hyperalgesia when thermal sensitivity was tested by measuring paw withdrawal to infrared heating, plantar test. The implications of test dependent magnitude of thermal hyperalgesia are discussed from the viewpoint of the tests used, as well as the animal models studied. It is argued that the dynamic thermal operant task reveals the relevance of the neuropathic injury associated pain-like behavior in relation to the whole organism.
Figures
Figure 1
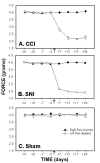
Mechanical sensitivity, force (g) required for 50% threshold for paw withdrawal, as a function of time in CCI (A), SNI (B) and sham (C) rats. Mechanical paw-withdrawal thresholds of the ligated paw were significantly attenuated after ligation (indicated by an arrow) in the CCI and SNI models as compared to the right paw (control), and to sham. In both groups, increased mechanical sensitivity persisted for 24 days after ligation.
Figure 2
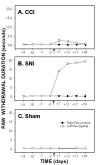
Cold sensitivity, paw-withdrawal duration to acetone, as a function of time in CCI (A), SNI (B) and sham (C) rats. Paw withdrawal duration to acetone applied to the ligated paw was significantly increased after ligation (indicated by an arrow) in SNI and CCI animals as compared to the right paw (control) and to sham. Increased cold sensitivity was maintained for 24 days after ligation in both groups. Cold sensitivity change was much smaller in CCI animals than in SNI rats.
Figure 3
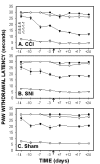
Plantar test for thermal sensitivity, paw-withdrawal latencies, as a function of time in CCI, SNI and sham rats, performed at 2 Infra Red (IR) intensities, 30 (A, B, and C) and 70 (D, E, and F). Paw withdrawal latencies of the ligated paw decreased in CCI and SNI rats 7 days post ligation as compared to the control (right) paw and to sham animals. Thermal hyperalgesia, as assessed on plantar test, was smaller in SNI than CCI rats.
Figure 4
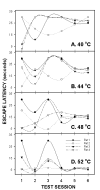
Hotplate test for thermal sensitivity, paw withdrawal latencies, as a function of time in CCI (A), SNI (B) and sham (C) rats, performed at 4 temperatures (40, 44, 48 and 52°C). Response latency decreases were most evident in CCI rats when tested at 48°C.
Figure 5
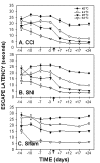
AlgoTrack test for thermal sensitivity, escape latencies, as a function of time in CCI (A), SNI (B) and sham (C) rats. The test was performed for all animals at 4 temperatures (40, 44, 48 and 52°C). Escape latencies were significantly reduced in CCI and SNI animals at all temperatures following ligation as compared to sham, and as compared to pre-ligation. The decrease in escape latencies was maximal at day 12 post-ligation and was maintained throughout the period of testing.
Figure 6
Comparison of the temperature-escape/paw withdrawal responses between AlgoTrack and Hotplate tests in CCI (A, D), SNI (B, E), and sham (C, F) animals tested at 3 days prior and at 7 and 17 days post-ligation. On the hotplate test, paw withdrawal latency decreases post-ligation are small and observed mainly in CCI rats. On the AlgoTrack test, both CCI (A) and SNI (B) rats exhibit post-ligation attenuation in their escape times at all temperatures tested.
Figure 7
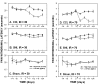
Comparing detection of thermal sensitivity changes between Plantar, Hotplate, and AlgoTrack tests in SNI rats (F-values in ANOVA planned comparisons). A) F-values for contrasting thermal pain behavior pre-ligation (3 days prior) to post-ligation (days 7, 17 and 24) on the Plantar test (P; for both 30 and 70 IR intensities); on the Hotplate test (H; for all test temperatures: 40, 44, 48, 52°C); and on the AlgoTrack test (for the same test temperatures). B) F-values for contrasting between SNI rats and sham rats on AlgoTrack test (gray bars) and Hotplate test (black bars) in post-ligation days (7, 17 and 24), for each applied temperature indicated. C) F-values for contrasting between SNI rats and sham rats on Plantar test in post-ligation days, for the IR intensities indicated. The y-axis is in log scale and covers 4 decades. The dashed line is for F = 2.0, which delineates threshold for significance.
Figure 8
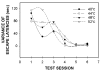
AlgoTrack escape latency variance in sham rats (n = 8) for 4 temperatures (40, 44, 48 and 52°C) as a function of test session. Variances for all temperatures tested decreased by the fourth testing session, and were maintained through further testing.
Figure 9
Algotrack individual responses of 4 sham rats for 4 temperatures 40 (A), 44 (B), 48 (C) and 52°C (D) plotted against test session. Illustrated are the first 6 sessions these animals were ever exposed to this paradigm. Each animal exhibits its own pattern of learning throughout the first few testing sessions, and then stabilizes to well-defined escape latencies for the different temperatures.
Similar articles
- Detection of cold pain, cold allodynia and cold hyperalgesia in freely behaving rats.
Allchorne AJ, Broom DC, Woolf CJ. Allchorne AJ, et al. Mol Pain. 2005 Dec 14;1:36. doi: 10.1186/1744-8069-1-36. Mol Pain. 2005. PMID: 16354295 Free PMC article. - Inflammatory and neuropathic pain animals exhibit distinct responses to innocuous thermal and motoric challenges.
Jabakhanji R, Foss JM, Berra HH, Centeno MV, Apkarian AV, Chialvo DR. Jabakhanji R, et al. Mol Pain. 2006 Jan 5;2:1. doi: 10.1186/1744-8069-2-1. Mol Pain. 2006. PMID: 16393346 Free PMC article. - Spared nerve injury: an animal model of persistent peripheral neuropathic pain.
Decosterd I, Woolf CJ. Decosterd I, et al. Pain. 2000 Aug;87(2):149-158. doi: 10.1016/S0304-3959(00)00276-1. Pain. 2000. PMID: 10924808 - Evaluating pain behaviours: Widely used mechanical and thermal methods in rodents.
Modi AD, Parekh A, Pancholi YN. Modi AD, et al. Behav Brain Res. 2023 May 28;446:114417. doi: 10.1016/j.bbr.2023.114417. Epub 2023 Mar 30. Behav Brain Res. 2023. PMID: 37003494 Review. - [Animal models of neuropathic pain].
Sasaki A, Kuraishi Y. Sasaki A, et al. Nihon Yakurigaku Zasshi. 2006 Mar;127(3):151-5, 146. doi: 10.1254/fpj.127.151. Nihon Yakurigaku Zasshi. 2006. PMID: 16651794 Review. Japanese. No abstract available.
Cited by
- Analogous responses in the nucleus accumbens and cingulate cortex to pain onset (aversion) and offset (relief) in rats and humans.
Becerra L, Navratilova E, Porreca F, Borsook D. Becerra L, et al. J Neurophysiol. 2013 Sep;110(5):1221-6. doi: 10.1152/jn.00284.2013. Epub 2013 Jun 19. J Neurophysiol. 2013. PMID: 23785130 Free PMC article. - Effects of decompression on behavioral, electrophysiologic, and histomorphologic recovery in a chronic sciatic nerve compression model of streptozotocin-induced diabetic rats.
Wang PH, Yang CC, Su WR, Wu PT, Cheng SC, Jou IM. Wang PH, et al. J Pain Res. 2017 Mar 20;10:643-652. doi: 10.2147/JPR.S125693. eCollection 2017. J Pain Res. 2017. PMID: 28360533 Free PMC article. - Effects of morphine on thermal sensitivity in adult and aged rats.
Morgan D, Mitzelfelt JD, Koerper LM, Carter CS. Morgan D, et al. J Gerontol A Biol Sci Med Sci. 2012 Jun;67(7):705-13. doi: 10.1093/gerona/glr210. Epub 2011 Dec 21. J Gerontol A Biol Sci Med Sci. 2012. PMID: 22193548 Free PMC article. - Thermal escape box: A cost-benefit evaluation paradigm for investigating thermosensation and thermal pain.
Dayton JR, Marquez J, Romo AK, Chen YJ, Contreras JE, Griffith TN. Dayton JR, et al. Neurobiol Pain. 2024 Apr 1;15:100155. doi: 10.1016/j.ynpai.2024.100155. eCollection 2024 Jan-Jun. Neurobiol Pain. 2024. PMID: 38617105 Free PMC article. - Efficacy of a Combination of N-Palmitoylethanolamide, Beta-Caryophyllene, Carnosic Acid, and Myrrh Extract on Chronic Neuropathic Pain: A Preclinical Study.
Fotio Y, Aboufares El Alaoui A, Borruto AM, Acciarini S, Giordano A, Ciccocioppo R. Fotio Y, et al. Front Pharmacol. 2019 Jun 27;10:711. doi: 10.3389/fphar.2019.00711. eCollection 2019. Front Pharmacol. 2019. PMID: 31316381 Free PMC article.
References
- Franklin KBJ, Abbott FV. Techniques for assessing the effects of drugs on nociceptive responses. In: Boultoun M, Baker GB and Greenshaw AJ, editor. Neuromethods, Psychopharmacology. Clifton, The Humana Press; 1989. pp. 145–215.
- Caroll MN, LIM RK. Observations on the neuropharmacology of morphine and morphinelike analgesia. Arch Int Pharmacodyn Ther. 1960;125:383–403. - PubMed
- Berridge KC. Progressive degradation of serial grooming chains by descending decerebration. Behav Brain Res. 1989;33:241–253. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical