Distinct effectors of platelet-derived growth factor receptor-alpha signaling are required for cell survival during embryogenesis - PubMed (original) (raw)
Distinct effectors of platelet-derived growth factor receptor-alpha signaling are required for cell survival during embryogenesis
Melanie Van Stry et al. Proc Natl Acad Sci U S A. 2005.
Abstract
Platelet-derived growth factor receptor (PDGFR) signaling is essential for normal embryonic development in many organisms, including frog, mouse, zebrafish, and sea urchin. The mode of action of PDGFR signaling during early development is poorly understood, however, mostly because inhibition of signaling through either the PDGFRalpha or PDGFRbeta is embryonic lethal. In Xenopus embryos, disruption of PDGFRalpha signaling causes migrating anterior mesoderm cells to lose direction and undergo apoptosis through the mitochondrial pathway. To understand the mechanism of PDGFRalpha function in this process, we have analyzed all known effector-binding sites in vivo. By using a chemical inducer of dimerization to activate chimera PDGFRalphas, we have identified a role for phospholipase Cgamma (PLCgamma) in protecting cells from death. PDGFRalpha-mediated cell survival requires PLCgamma and phosphatidylinositol 3-kinase signaling, and that PDGFRalpha with binding sites for these two signaling factors is sufficient for this activity. Other effectors of PDGFRalpha signaling, Shf, SHP-2, and Crk, are not required for this process. Thus, our findings show that PDGFRalpha signaling through PLCgamma and phosphatidylinositol 3-kinase has a protective role in preventing apoptosis in early development. Furthermore, we demonstrate that small molecule inducers of dimerization provide a powerful system to manipulate receptor function in developing embryos.
Figures
Fig. 1.
Schematic of _i_PDGFRα mutants. To dissect PDGFRα signaling, tyrosines that when phosphorylated (P) bind and activate specific downstream effectors were replaced by phenylalanine (black squares) by site-directed mutagenesis. (A) Subtraction mutants contain mutations that allow binding and activation of all but one downstream effector. (B) Add-back mutants contain mutations to allow binding and activation of one or more downstream effector. (C) _i_PDGFRα is a fusion protein of the myristoylation signal from v-Src, three tandem repeats of FKBP12 containing point mutations G89P and I90K to block calcineurin binding, and the cytoplasmic domain of the PDGFRα with or without specific Y→F mutations. The addition of the dimerizer, AP1510, activates the receptor kinase through the induced dimerization of two of the receptor fusion proteins.
Fig. 2.
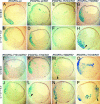
PDGFR signaling through PLCγ and PI3K, but not through SHP-2, Shf, and Crk, is required for mesoderm cell survival. (A and B) Embryos were coinjected with mRNA encoding β-gal and _i_PDGFRα or β-gal, PDGFR-37 (R37), and the following _i_PDGFRαs. (C and D) wt. (E and F) F572/74. (G and H) F720. (I and J) F731/42. (K and L) F762. (M and N) F988. (O and P) F1018. At the beginning of gastrulation (stage 10), AP1510 or DMSO was injected into the blastocoel. At the midgastrula stage (stage 11), β-gal expression was visualized (shown in blue). The stained embryos were dissected and scored for the presence or absence of nonnuclear β-gal-stained cells in the blastocoel cavity, within the vitelline membrane, or in the process of being excluded from the embryo (see red arrowhead in K), indicating the presence of apoptotic cells. The percentage of embryos containing apoptotic cells was calculated. Representative saggital sections of these embryos are shown. (G–P) Note that when signaling through PLCγ and PI3K is prevented (I, J, and M_–_P), activation of the receptor with AP1510 did not restore cell survival, whereas cell survival is restored when signaling through SHP-2, Shf, and Crk is prevented (G, H, K, and L). Arrowheads indicate apoptotic mesoderm cells outside the blastocoel cavity. Note that overexpression of _i_PDGFRα-wt mRNA alone does not cause apoptosis in mesoderm cells.
Fig. 3.
PDGFR signaling through a single downstream effector is not sufficient for mesoderm cell survival. (A and B) Embryos were coinjected with mRNA encoding β-gal and _i_PDGFRα or β-gal, PDGFR-37 (R37), and the following _i_PDGFRαs. (C and D) wt. (E and F) Y572/74. (G and H) Y720. (I and J) Y731/42. (K and L) Y762. (M and N) Y988. (O and P) Y1018. At the beginning of gastrulation (stage 10), AP1510 or DMSO was injected into the blastocoel. At the midgastrula stage (stage 11), β-gal expression was visualized (shown in blue). Representative saggital sections of these embryos are shown. (E_–_P) Note that single effectors do not restore cell survival. Arrowheads indicate apoptotic mesoderm cells outside the blastocoel cavity.
Fig. 4.
PDGFR signaling through PLCγ and PI3K is required for mesoderm cell survival. (A and B) Embryos were coinjected with mRNA encoding β-gal and _i_PDGFRα or β-gal, PDGFR-37 (R37), and the following _i_PDGFRαs. (C and D) wt. (E and F) F720/762. (G and H) F4. At the beginning of gastrulation (stage 10), AP1510 or DMSO was injected into the blastocoel. At the midgastrula stage (stage 11), β-gal expression was visualized (shown in blue). Representative saggital sections of these embryos are shown. As shown in E and F, only the presence of PLCγ, PI3K, and Src pYBs are required to restore mesoderm cell survival.
Fig. 5.
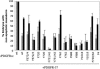
PDGFR signaling through PLCγ and PI3K is required for mesoderm cell survival. The data presented in Figs. 2, 3, 4 are shown in graph form. The percentage of embryos that do not contain apoptotic cells (i.e., cells with nonnuclear β-gal staining) is presented for embryos injected with mRNA as described in Figs. 2, 3, 4 and with DMSO (gray bars) or AP1510 (black bars). This percentage is low for some mutants compared with wt because there may be some basal activity of the receptor construct without the addition of dimerizer. Error bars represent standard error and were calculated from a minimum of three separate experiments. The data used to construct this graph is available in Table 1, which is published as
supporting information
on the PNAS web site.
Similar articles
- Disruption of gap junctional communication by the platelet-derived growth factor is mediated via multiple signaling pathways.
Hossain MZ, Jagdale AB, Ao P, Kazlauskas A, Boynton AL. Hossain MZ, et al. J Biol Chem. 1999 Apr 9;274(15):10489-96. doi: 10.1074/jbc.274.15.10489. J Biol Chem. 1999. PMID: 10187840 - Differential tyrosine phosphorylation of fibroblast growth factor (FGF) receptor-1 and receptor proximal signal transduction in response to FGF-2 and heparin.
Lundin L, Rönnstrand L, Cross M, Hellberg C, Lindahl U, Claesson-Welsh L. Lundin L, et al. Exp Cell Res. 2003 Jul 1;287(1):190-8. doi: 10.1016/s0014-4827(03)00125-3. Exp Cell Res. 2003. PMID: 12799194 - Phosphorylation of tyrosine 720 in the platelet-derived growth factor alpha receptor is required for binding of Grb2 and SHP-2 but not for activation of Ras or cell proliferation.
Bazenet CE, Gelderloos JA, Kazlauskas A. Bazenet CE, et al. Mol Cell Biol. 1996 Dec;16(12):6926-36. doi: 10.1128/MCB.16.12.6926. Mol Cell Biol. 1996. PMID: 8943348 Free PMC article. - Signaling by Kit protein-tyrosine kinase--the stem cell factor receptor.
Roskoski R Jr. Roskoski R Jr. Biochem Biophys Res Commun. 2005 Nov 11;337(1):1-13. doi: 10.1016/j.bbrc.2005.08.055. Biochem Biophys Res Commun. 2005. PMID: 16129412 Review.
Cited by
- SHF Acts as a Novel Tumor Suppressor in Glioblastoma Multiforme by Disrupting STAT3 Dimerization.
Wang J, Huang Z, Ji L, Chen C, Wan Q, Xin Y, Pu Z, Li K, Jiao J, Yin Y, Hu Y, Gong L, Zhang R, Yang X, Fang X, Wang M, Zhang B, Shao J, Zou J. Wang J, et al. Adv Sci (Weinh). 2022 Sep;9(26):e2200169. doi: 10.1002/advs.202200169. Epub 2022 Jul 17. Adv Sci (Weinh). 2022. PMID: 35843865 Free PMC article. - SHP-2-upregulated ZEB1 is important for PDGFRα-driven glioma epithelial-mesenchymal transition and invasion in mice and humans.
Zhang L, Zhang W, Li Y, Alvarez A, Li Z, Wang Y, Song L, Lv D, Nakano I, Hu B, Cheng SY, Feng H. Zhang L, et al. Oncogene. 2016 Oct 27;35(43):5641-5652. doi: 10.1038/onc.2016.100. Epub 2016 Apr 4. Oncogene. 2016. PMID: 27041571 Free PMC article. - Pdgfra protects against ethanol-induced craniofacial defects in a zebrafish model of FASD.
McCarthy N, Wetherill L, Lovely CB, Swartz ME, Foroud TM, Eberhart JK. McCarthy N, et al. Development. 2013 Aug;140(15):3254-65. doi: 10.1242/dev.094938. Development. 2013. PMID: 23861062 Free PMC article. - Control of gastruloid patterning and morphogenesis by the Erk and Akt signaling pathways.
Underhill EJ, Toettcher JE. Underhill EJ, et al. Development. 2023 Aug 15;150(16):dev201663. doi: 10.1242/dev.201663. Epub 2023 Aug 17. Development. 2023. PMID: 37590131 Free PMC article. - Reversal of experimental pulmonary hypertension by PDGF inhibition.
Schermuly RT, Dony E, Ghofrani HA, Pullamsetti S, Savai R, Roth M, Sydykov A, Lai YJ, Weissmann N, Seeger W, Grimminger F. Schermuly RT, et al. J Clin Invest. 2005 Oct;115(10):2811-21. doi: 10.1172/JCI24838. J Clin Invest. 2005. PMID: 16200212 Free PMC article.
References
- Hoch, R. V. & Soriano, P. (2003) Development (Cambridge, U.K.) 130, 4769-4784. - PubMed
- Soriano, P. (1997) Development (Cambridge, U.K.) 124, 2691-2700. - PubMed
- Ataliotis, P., Symes, K., Chou, M. M., Ho, L. & Mercola, M. (1995) Development (Cambridge, U.K.) 121, 3099-3110. - PubMed
- Nagel, M., Tahinci, E., Symes, K. & Winklbauer, R. (2004) Development (Cambridge, U.K.) 131, 2727-2736. - PubMed
- Van Stry, M., McLaughlin, K. A., Ataliotis, P. & Symes, K. (2004) Dev. Biol. 268, 232-242. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA087375/CA/NCI NIH HHS/United States
- EY012509/EY/NEI NIH HHS/United States
- Z01 AG000115/ImNIH/Intramural NIH HHS/United States
- R01 CA087375-03/CA/NCI NIH HHS/United States
- R01 EY012509/EY/NEI NIH HHS/United States
- T32 AG000115/AG/NIA NIH HHS/United States
- R01 CA087375-02/CA/NCI NIH HHS/United States
- CA87375/CA/NCI NIH HHS/United States
- R01 CA087375-04/CA/NCI NIH HHS/United States
- R01 CA087375-01/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous