STAT3 nuclear import is independent of tyrosine phosphorylation and mediated by importin-alpha3 - PubMed (original) (raw)
STAT3 nuclear import is independent of tyrosine phosphorylation and mediated by importin-alpha3
Ling Liu et al. Proc Natl Acad Sci U S A. 2005.
Abstract
Signal transducer and activator of transcription (STAT)3 is a member of a family of DNA-binding factors that function to induce expression of responsive genes. STAT3 can act as an oncogene, and its function has been shown to be critical for cellular transformation by a number of oncogenic tyrosine kinases. The role of STAT3 as a DNA-binding transcription factor naturally depends on its ability to gain entrance to the nucleus. In this study, we provide evidence that STAT3 is distinct from previously characterized STAT molecules in that it dynamically shuttles between cytoplasmic and nuclear compartments and maintains prominent nuclear presence. Although tyrosine phosphorylation is required for STAT3 to bind to specific DNA target sites, nuclear import takes place constitutively and independently of tyrosine phosphorylation. We identify a region within the coiled-coil domain of the STAT3 molecule that is necessary for nuclear import and demonstrate that this region is critical for its recognition by specific import carrier importin-alpha3. RNA interference studies were used to verify the role and specificity of importin-alpha3 in STAT3 nuclear translocation. These results distinguish STAT3 cellular localization from other STAT molecules and identify a feature that may be targeted in the clinical intervention of STAT3-dependent neoplasia.
Figures
Fig. 1.
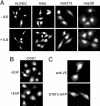
Constitutive nuclear presence of STAT3 in various cell lines. Images represent >95% of the cell population. (A) Immunofluorescence of endogenous STAT3 in human umbilical cord vein endothelial cells (HUVEC), HeLa, NIH-3T3 and Hep3B cells that were serum-starved (-IL6) or treated with IL-6 for 30 min (+IL6). Cells were stained with polyclonal anti-STAT3 (Cell Signaling Technology) followed by rhodamine-conjugated secondary antibodies. (B) Immunofluorescence of endogenous STAT3 in COS1 cells serum-starved (-EGF) or treated with EGF for 30 min (+EGF) and stained as in A. (C) Cells expressing V5-STAT3 were stained with monoclonal anti-V5 antibody followed by rhodamine-conjugated secondary antibodies. Cells expressing STAT3–GFP were processed for direct fluorescence microscopy.
Fig. 2.
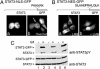
STAT3 nuclear translocation occurs independently of tyrosine phosphorylation. (A) HeLa cells (lanes 1 and 2) and HeLa cells transfected with STAT3–GFP (lanes 3 and 4) were untreated (-) or treated (+) with human IL-6 for 30 min. Whole-cell lysates were subjected to SDS/PAGE and Western blot with specific anti-STAT3 phosphotyrosine antibody (Left) or anti-STAT3 antibody (Right). (B) HeLa cells were transfected with wild-type STAT3–GFP or STAT3-RY–GFP, and localization of the proteins was examined by fluorescence microscopy. More than 95% of the transfected cells exhibited localization of the STAT3–GFP fusion as shown in the images.
Fig. 3.
Nuclear–cytoplasmic shuttling of STAT3. (A) HeLa cells expressing STAT3–NLS–GFP were fused with NIH-3T3 cells. (a) Localization of the STAT3–NLS–GFP in heterokaryons was examined by fluorescent microscopy. (b) Nuclei were distinguished with Hoechst stain. NIH-3T3 nucleus within a heterokaryon containing STAT3–NLS–GFP is noted with a characteristic punctate staining (arrow). (B) HeLa cells transfected with STAT3–NES–GFP were untreated (a) or treated (b) with 10 nM LMB for 15 min. Localization of the GFP fusion was examined by fluorescent microscopy. STAT3–NES–GFP accumulated in 100% of transfected cells in response to LMB treatment. (C) HeLa cells transfected with STAT3–GFP (lanes 1 and 2), STAT3–NES–GFP (lanes 3 and 4), or STAT3–NLS–GFP (lanes 5 and 6) were untreated (-) or treated (+) with human IL-6 for 30 min. Cell lysates were subjected to SDS/PAGE and Western blot with specific anti-STAT3 phosphotyrosine (Upper) and anti-STAT3 (Lower) antibodies.
Fig. 4.
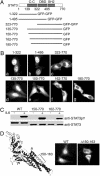
Cellular localization of STAT3 deletion mutations. (A) Linear diagram of STAT3 deletion constructs. (B) Localization of STAT3 domains fused with two tandem GFPs and STAT3 amino-terminal deletions fused with GFP. Images are representative of >90% of the cell population. (C) HeLa cells transfected with the STAT3–GFP, STAT3(150–770)–GFP, or STAT3(162–770)–GFP were untreated (-) or treated (+) with human IL-6 for 30 min. Cell lysates were subjected to SDS/PAGE and Western blot with specific anti-STAT3 phosphotyrosine (Upper) and anti-STAT3 (Lower) antibodies. (D) STAT3–GFP with an internal deletion of the amino acids 150–163 region (Δ150–163) is defective in nuclear import. (Left) Position of the amino acids 150–163 region in the crystal structure of STAT3β (Protein Data Bank ID code 1BG5) in a ribbon representation. (Center and Right) Cellular localization of the wild type (Center) and STAT3(Δ150–163)–GFP mutant (Right).
Fig. 5.
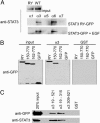
STAT3 binds to importin-α3 and importin-α6. (A) GST–importin-α(ΔIBB) proteins bound to glutathione agarose beads were incubated with lysate from COS cells transfected with STAT3-RY–GFP or cells transfected with wild-type STAT3–GFP and treated with EGF for 30 min. Interacting proteins were analyzed by Western blot using monoclonal STAT3 antibody. (B) GST–importin-α3(ΔIBB) or GST proteins bound to glutathione agarose beads were incubated with lysates from cells expressing GFP, STAT3-RY–GFP, STAT3(150–770)–GFP or STAT3(162–770)–GFP. Interacting proteins were analyzed by Western blot using anti-GFP antibody. (C) GST–importin-α3ΔIBB (amino acids 19–521), GST–importin-α3 central Armadillo repeats (amino acids 19–315), GST–importin-α3 carboxyl terminus (amino acids 309–521), or GST proteins were immobilized on glutathione agarose beads and incubated with lysates from COS1 cells expressing STAT3-RY–GFP (Upper) or from Sf9 insect cells (Lower) expressing His-6–STAT3-RY. Bound STAT3 proteins were detected by Western blot with anti-GFP antibody (Upper) or anti-STAT3 antibody (Lower).
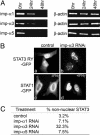
Fig. 6.
Effect of importin-α3 siRNA on STAT3 nuclear accumulation. (A) Total RNA was prepared from cells 0, 24, or 48 h after specific siRNA transfection. Reduction of mRNAs encoding importin-α1, importin-α3 or importin-α5 proteins was detected by quantitative real-time RT-PCR using specific primers. β-actin mRNA was amplified as internal control. (B) Images of cells expressing STAT3-RY–GFP in control cultures or after importin-α3 siRNA transfection (imp-α3 RNAi). Images of cells expressing STAT1–GFP in control cultures (-IFN-γ) or importin-α3 siRNA transfected cells after stimulation by IFN-γ (+IFN-γ). (C) Percentage of cells that exhibited aberrant STAT3-RY–GFP localization for each siRNA in this experiment. Results are representative of three independent experiments.
Similar articles
- Extracellular signal-dependent nuclear import of STAT3 is mediated by various importin alphas.
Ushijima R, Sakaguchi N, Kano A, Maruyama A, Miyamoto Y, Sekimoto T, Yoneda Y, Ogino K, Tachibana T. Ushijima R, et al. Biochem Biophys Res Commun. 2005 May 13;330(3):880-6. doi: 10.1016/j.bbrc.2005.03.063. Biochem Biophys Res Commun. 2005. PMID: 15809078 - Dissecting functions of the N-terminal domain and GAS-site recognition in STAT3 nuclear trafficking.
Martincuks A, Fahrenkamp D, Haan S, Herrmann A, Küster A, Müller-Newen G. Martincuks A, et al. Cell Signal. 2016 Aug;28(8):810-25. doi: 10.1016/j.cellsig.2016.03.011. Epub 2016 Mar 31. Cell Signal. 2016. PMID: 27040695 - Nuclear retention of STAT3 through the coiled-coil domain regulates its activity.
Sato N, Tsuruma R, Imoto S, Sekine Y, Muromoto R, Sugiyama K, Matsuda T. Sato N, et al. Biochem Biophys Res Commun. 2005 Oct 21;336(2):617-24. doi: 10.1016/j.bbrc.2005.08.145. Biochem Biophys Res Commun. 2005. PMID: 16140268 - The ins and outs of STAT1 nuclear transport.
McBride KM, Reich NC. McBride KM, et al. Sci STKE. 2003 Aug 12;2003(195):RE13. doi: 10.1126/stke.2003.195.re13. Sci STKE. 2003. PMID: 12915721 Review. - STATs get their move on.
Reich NC. Reich NC. JAKSTAT. 2013 Oct 1;2(4):e27080. doi: 10.4161/jkst.27080. Epub 2013 Nov 13. JAKSTAT. 2013. PMID: 24470978 Free PMC article. Review.
Cited by
- Nuclear import of aristaless-related homeobox protein via its NLS1 regulates its transcriptional function.
Ye W, Lin W, Tartakoff AM, Ma Q, Tao T. Ye W, et al. Mol Cell Biochem. 2013 Sep;381(1-2):221-31. doi: 10.1007/s11010-013-1706-7. Epub 2013 Jun 16. Mol Cell Biochem. 2013. PMID: 23771350 - Inhibition of the JAK2/STAT3 pathway and cell cycle re-entry contribute to the protective effect of remote ischemic pre-conditioning of rat hindlimbs on cerebral ischemia/reperfusion injury.
Zhao Y, Ding M, Yan F, Yin J, Shi W, Yang N, Zhao H, Fang Y, Huang Y, Zheng Y, Yang X, Li W, Ji X, Luo Y. Zhao Y, et al. CNS Neurosci Ther. 2023 Mar;29(3):866-877. doi: 10.1111/cns.14023. Epub 2022 Nov 23. CNS Neurosci Ther. 2023. PMID: 36419252 Free PMC article. - Model-based extension of high-throughput to high-content data.
Pfeifer AC, Kaschek D, Bachmann J, Klingmüller U, Timmer J. Pfeifer AC, et al. BMC Syst Biol. 2010 Aug 5;4:106. doi: 10.1186/1752-0509-4-106. BMC Syst Biol. 2010. PMID: 20687942 Free PMC article. - Targeted inhibition of STAT3 as a potential treatment strategy for atherosclerosis.
Chen Q, Lv J, Yang W, Xu B, Wang Z, Yu Z, Wu J, Yang Y, Han Y. Chen Q, et al. Theranostics. 2019 Aug 14;9(22):6424-6442. doi: 10.7150/thno.35528. eCollection 2019. Theranostics. 2019. PMID: 31588227 Free PMC article. Review. - The Role of Signal Transducer and Activator of Transcription 3 (STAT3) and Its Targeted Inhibition in Hematological Malignancies.
Arora L, Kumar AP, Arfuso F, Chng WJ, Sethi G. Arora L, et al. Cancers (Basel). 2018 Sep 13;10(9):327. doi: 10.3390/cancers10090327. Cancers (Basel). 2018. PMID: 30217007 Free PMC article. Review.
References
- Akira, S. (1999) Stem Cells 17, 138-146. - PubMed
- Bromberg, J. & Darnell, J. E., Jr. (2000) Oncogene 19, 2468-2473. - PubMed
- Darnell, J. E., Jr. (1997) Science 277, 1630-1635. - PubMed
- Ihle, J. N. (2001) Curr. Opin. Cell Biol. 13, 211-217. - PubMed
- Davis, L. I. (1995) Annu. Rev. Biochem. 64, 865-896. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous