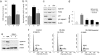Cell cycle inhibition provides neuroprotection and reduces glial proliferation and scar formation after traumatic brain injury - PubMed (original) (raw)
Cell cycle inhibition provides neuroprotection and reduces glial proliferation and scar formation after traumatic brain injury
Simone Di Giovanni et al. Proc Natl Acad Sci U S A. 2005.
Abstract
Traumatic brain injury (TBI) causes neuronal apoptosis, inflammation, and reactive astrogliosis, which contribute to secondary tissue loss, impaired regeneration, and associated functional disabilities. Here, we show that up-regulation of cell cycle components is associated with caspase-mediated neuronal apoptosis and glial proliferation after TBI in rats. In primary neuronal and astrocyte cultures, cell cycle inhibition (including the cyclin-dependent kinase inhibitors flavopiridol, roscovitine, and olomoucine) reduced up-regulation of cell cycle proteins, limited neuronal cell death after etoposide-induced DNA damage, and attenuated astrocyte proliferation. After TBI in rats, flavopiridol reduced cyclin D1 expression in neurons and glia in ipsilateral cortex and hippocampus. Treatment also decreased neuronal cell death and lesion volume, reduced astroglial scar formation and microglial activation, and improved motor and cognitive recovery. The ability of cell cycle inhibition to decrease both neuronal cell death and reactive gliosis after experimental TBI suggests that this treatment approach may be useful clinically.
Figures
Fig. 1.
Cell cycle and DNA damage-related mRNA and protein changes after TBI. (a) Shown is a dendrogram (gene tree) obtained nucleating altered transcripts between sham and injured brains. Bar graph (Right) shows the color code for gene expression levels from low (blue) to high (red). These transcripts are up-regulated between 4 and 72 h after TBI (gene names in yellow indicate a Welch t test P value <0.05 between sham and injured at one corresponding time point). (b) Table shows gene name, accession number, and quantitation (fold changes) of mRNA changes by Affymetrix for the transcripts represented in the dendrogram. Genes with asterisks indicate a Welch t test P value <0.05 between sham and injured at the corresponding time point. (c) RT-PCR confirms expression of mRNAs for GADD45, PCNA, c-Myc, and p27 (ribosomal protein L-19 was used as control). (d) Immunoblots show increased GADD45 and PCNA protein levels in injured (I), versus sham (S) brains at 24 h in two parallel experiments. β-Actin is a loading control. (e) Immunoblotting at 72 h shows induction of cyclin D1 and inhibition of p27 protein expression after TBI in injured (I) versus sham (S) brains; flavopiridol (F) rescues p27 expression and inhibits cyclin D1. β-Actin is a loading control.
Fig. 2.
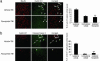
Flavopiridol promotes neuronal survival after DNA damage and inhibits astrocyte proliferation by blocking cell cycle proteins and cell cycle progression. (a and b) Flavopiridol treatment protects cells from death induced by etoposide (LDH release) (a) and number of Hoechst-positive cells (b) in primary cortical neurons at 24 h. (c) Immunoblotting shows expression of cell cycle proteins and caspase 3 after etoposide treatment, and etoposide plus flavopiridol treatment from rat cortical neuronal cells after treatment with etoposide (lane E) and after etoposide plus flavopiridol (lane E+F) at 24 h. (d) Flavopiridol blocks proliferation of cultured rat astrocytes. Shown are data from MTT cell viability assay in astrocytes after serum stimulation and treatment with flavopiridol. Values on y axis are expressed in OD, reflecting number of viable cells. (e) Flavopiridol completely suppresses cyclin D1 and PCNA protein expression in proliferating astrocytes. Immunoblotting at 48 h after serum stimulation (lane FBS) shows induction of cyclin D1 and PCNA compared with control resting astrocytes (lane C). Flavopiridol in duplicate experiments, administered 1 h before FBS completely suppresses cyclin D1 and PCNA protein expression (lanes FBS+F). (f) Flavopiridol reduces S phase in proliferating astrocytes to basal levels (FACS).
Fig. 3.
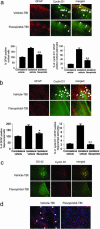
Immunofluorescence shows the expression of cyclin D1 and active caspase 3 in cortical neurons after TBI, and effects of flavopiridol treatment. (a) Double immunofluorescence in coronal sections of the brain cortex around the injury site shows staining for cyclin D1 in vehicle, and flavopiridol-treated animal after TBI, and for the neuronal marker NeuN. Cyclin D1 expression is induced in cortical neurons after TBI and strongly reduced after flavopiridol compared with vehicle (arrows). (Original magnification: ×125.) Bar graphs show quantitation of cortical neurons expressing cyclin D1 in ipsilateral and contralateral cortex in vehicle-treated rats, compared with flavopiridol (ipsilateral) (**, P < 0.01). (b) Double immunofluorescence in coronal sections of the brain cortex around the injury site shows staining for cyclin D1 in vehicle, and flavopiridol-treated animal after TBI, and for active caspase 3. Flavopiridol strongly reduces cyclin D1-positive and active caspase 3-positive neurons (arrows). (Original magnification: ×125.) Bar graphs show quantitation of cortical neurons coexpressing cyclin D1 and cleaved caspase 3 in ipsilateral and contralateral cortex in vehicle-treated rats, compared with flavopiridol (ipsilateral) (**, P < 0.01).
Fig. 4.
Immunofluorescence in glial cells after TBI and flavopiridol treatment. (a) Double immunofluorescence in coronal sections of brain cortex around the injury site shows staining for cyclin D1 and for astrocyte marker GFAP in vehicle, and flavopiridol-treated animals after TBI. Strong reduction of cyclin D1-positive and GFAP-positive cells is present after flavopiridol compared with vehicle (arrows). (Original magnification: ×125.) Bar graphs show quantitation of these data (**, P < 0.01). (b) Double immunofluorescence in coronal sections of hippocampus (CA1 and CA2) on the injury site shows staining for cyclin D1 and GFAP in vehicle, and flavopiridol-treated animal after TBI. Strong reduction of cyclin D1-positive and GFAP-positive cells is present after flavopiridol compared with vehicle (arrows). (Original magnification: ×125.) Bar graphs show quantitation of these data (*, P < 0.05; **, P < 0.01). (c) Double immunofluorescence in coronal sections of brain cortex around the injury site shows staining for cyclin D1 and for activated microglia marker OX-42 in vehicle, and flavopiridol-treated animals after TBI. Just sporadic GFAP-positive cells are present after flavopiridol (arrows). (Original magnification: ×125.) (d) Immunostaining for GFAP and nuclear marker (TOPRO) in ipsilateral cortex after TBI, comparing vehicle and flavopiridol-treated rats at 28 days posttrauma. Flavopiridol (250 μM) limits the injury-induced reactive gliosis and tissue loss. (Original magnification: ×125.)
Fig. 5.
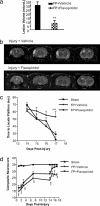
Flavopiridol significantly reduces lesion volume 21 days after TBI in rats. (a) Bar graph shows significant reduction of lesion volume 21 days after TBI (30 min after the injury, at 250 μmol i.c.v.; n = 7; lesion volumes: mean ± SEM); (**, P < 0.01 vs. FP plus vehicle). (b) Representative T2-weighted MRI of vehicle and flavopiridol-treated rats shows marked reduction of lesion volume. (c) Flavopiridol improves cognitive performance as measured by the Morris water maze test (***, P < 0.001 compared with sham control animals). (d) Flavopiridol restores motor function close to control levels. Results are shown as daily mean ± SEM (**, P < 0.01 and ***, P < 0.001 vs. sham controls).
Similar articles
- Roscovitine reduces neuronal loss, glial activation, and neurologic deficits after brain trauma.
Hilton GD, Stoica BA, Byrnes KR, Faden AI. Hilton GD, et al. J Cereb Blood Flow Metab. 2008 Nov;28(11):1845-59. doi: 10.1038/jcbfm.2008.75. Epub 2008 Jul 9. J Cereb Blood Flow Metab. 2008. PMID: 18612315 Free PMC article. - Role of the cell cycle in the pathobiology of central nervous system trauma.
Cernak I, Stoica B, Byrnes KR, Di Giovanni S, Faden AI. Cernak I, et al. Cell Cycle. 2005 Sep;4(9):1286-93. doi: 10.4161/cc.4.9.1996. Epub 2005 Sep 15. Cell Cycle. 2005. PMID: 16082214 - CR8, a novel inhibitor of CDK, limits microglial activation, astrocytosis, neuronal loss, and neurologic dysfunction after experimental traumatic brain injury.
Kabadi SV, Stoica BA, Loane DJ, Luo T, Faden AI. Kabadi SV, et al. J Cereb Blood Flow Metab. 2014 Mar;34(3):502-13. doi: 10.1038/jcbfm.2013.228. Epub 2014 Jan 8. J Cereb Blood Flow Metab. 2014. PMID: 24398934 Free PMC article. - Cell cycle activation and spinal cord injury.
Wu J, Stoica BA, Faden AI. Wu J, et al. Neurotherapeutics. 2011 Apr;8(2):221-8. doi: 10.1007/s13311-011-0028-2. Neurotherapeutics. 2011. PMID: 21373950 Free PMC article. Review. - Protective mechanisms of adenosine in neurons and glial cells.
Schubert P, Ogata T, Marchini C, Ferroni S, Rudolphi K. Schubert P, et al. Ann N Y Acad Sci. 1997 Oct 15;825:1-10. doi: 10.1111/j.1749-6632.1997.tb48409.x. Ann N Y Acad Sci. 1997. PMID: 9369970 Review.
Cited by
- Loss of the androgen receptor cofactor p44/WDR77 induces astrogliosis.
Vincent B, Wu H, Gao S, Wang Z. Vincent B, et al. Mol Cell Biol. 2012 Sep;32(17):3500-12. doi: 10.1128/MCB.00298-12. Epub 2012 Jul 2. Mol Cell Biol. 2012. PMID: 22751923 Free PMC article. - Increased expression of BAG-1 in rat brain cortex after traumatic brain injury.
Xu T, Wang X, Cao M, Wu X, Yan Y, Fu H, Zhao W, Gong P, Ke K, Gu X. Xu T, et al. J Mol Histol. 2012 Jun;43(3):335-42. doi: 10.1007/s10735-012-9408-0. Epub 2012 Apr 13. J Mol Histol. 2012. PMID: 22526508 - Dynasore Improves Motor Function Recovery via Inhibition of Neuronal Apoptosis and Astrocytic Proliferation after Spinal Cord Injury in Rats.
Li G, Shen F, Fan Z, Wang Y, Kong X, Yu D, Zhi X, Lv G, Cao Y. Li G, et al. Mol Neurobiol. 2017 Nov;54(9):7471-7482. doi: 10.1007/s12035-016-0252-1. Epub 2016 Nov 7. Mol Neurobiol. 2017. PMID: 27822712 - Melatonin as a Harmonizing Factor of Circadian Rhythms, Neuronal Cell Cycle and Neurogenesis: Additional Arguments for Its Therapeutic Use in Alzheimer's Disease.
Shukla M, Vincent B. Shukla M, et al. Curr Neuropharmacol. 2023;21(5):1273-1298. doi: 10.2174/1570159X21666230314142505. Curr Neuropharmacol. 2023. PMID: 36918783 Free PMC article. Review. - Nurr1 Modulation Mediates Neuroprotective Effects of Statins.
Willems S, Marschner JA, Kilu W, Faudone G, Busch R, Duensing-Kropp S, Heering J, Merk D. Willems S, et al. Adv Sci (Weinh). 2022 Jun;9(18):e2104640. doi: 10.1002/advs.202104640. Epub 2022 Apr 30. Adv Sci (Weinh). 2022. PMID: 35488520 Free PMC article.
References
- McGraw, J., Hiebert, G. W. & Steeves, J. D. (2001) J. Neurosci. Res. 63, 109-115. - PubMed
- Yakovlev, A. G. & Faden, A. I. (2001) Mol. Neurobiol. 24, 131-144. - PubMed
- Liou, A. K., Clark, R. S., Henshall, D. C., Yin, X. M. & Chen, J. T. (2003) Prog. Neurobiol. 69, 103-142. - PubMed
- Faden, A. I. (2002) Curr. Opin. Neurol. 15, 707-712. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials