Mitochondrial reactive oxygen species activation of p38 mitogen-activated protein kinase is required for hypoxia signaling - PubMed (original) (raw)
Mitochondrial reactive oxygen species activation of p38 mitogen-activated protein kinase is required for hypoxia signaling
Brooke M Emerling et al. Mol Cell Biol. 2005 Jun.
Abstract
Mammalian cells have the ability to sense low oxygen levels (hypoxia). An adaptive response to hypoxia involves the induction of the transcription factor hypoxia-inducible factor 1 (HIF-1). The intracellular signaling pathways that regulate HIF-1 activation during hypoxia remain unknown. Here, we demonstrate that p38alpha-/- cells fail to activate HIF-1 under hypoxic conditions. Cells deficient in Mkk3 and Mkk6, the upstream regulators of p38alpha, also fail to activate HIF-1 under hypoxic conditions. The p38alpha-/- cells are able to activate HIF-1 in response to anoxia or iron chelators during normoxia. Furthermore, the hypoxic activation of p38alpha and HIF-1 was abolished by myxothiazol, a mitochondrial complex III inhibitor, and glutathione peroxidase 1 (GPX1), a scavenger of hydrogen peroxide. Thus, the activation of p38alpha and HIF-1 is dependent on the generation of mitochondrial reactive oxygen species. These results provide genetic evidence that p38 mitogen-activated protein kinase signaling is essential for HIF-1 activation.
Figures
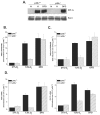
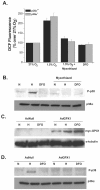
FIG. 1.
p38α MAPK is required for hypoxic activation of HIF-1. (A) HIF-1α protein levels in p38α +/+ and _p38α_−/− cells exposed to 21% O2 (N) ±100 μM DFO or to 1.5% O2 (H) for 2 h. (B) p38α +/+ and _p38α_−/− cells transfected with the HRE-Luciferase reporter gene construct and exposed to 21% O2 ± 100 μM DFO or to 1.5% O2 for 16 h. (C) p38α +/+ and _p38α_−/− cells transfected with a GAL4 (1 to 147) DNA-binding domain fused to HIF-1α (531 to 826) construct and a reporter gene construct encoding five GAL4-binding sites and exposed to 21% O2 ± 100 μM DFO or to 1.5% O2 for 36 h. (D) p38α +/+ and _p38α_−/− cells cultured for 16 h under 21% O2 ± 100 μM DFO or to 1.5% O2, and transcription levels of pgk1 and glut1 were determined by quantitative real-time RT-PCR analysis.
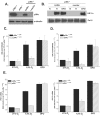
FIG. 2.
p38α MAPK is not required for anoxic activation of HIF-1. (A) HIF-1α protein levels in p38α +/+ and _p38α_−/− cells exposed to 21% O2 (N) or to 0.0% O2 (A) for 2 h. (B) p38α +/+ and _p38α_−/− cells transfected with the HRE-Luciferase reporter gene construct and exposed to 21% O2 or to 0.0% O2 for 16 h.
FIG. 3.
Reintroduction of p38α rescues hypoxic activation of HIF-1 in p38α null cells. (A) p38α_−/− cells stably reconstituted with a p38α cDNA or with vector alone. (B) HIF-1α protein levels in reconstituted cells exposed to 21% O2 (N) ± 100 μM DFO or to 1.5% O2 (H) for 2 h. (C) Cells transfected with HRE-Luciferase and exposed to 21% O2 ± 100 μM DFO or to 1.5% O2 for 16 h. (D) Cells transfected with a GAL4 (1 to 147) DNA-binding domain fused to HIF-1α (531 to 826) construct and a reporter gene construct encoding five GAL4-binding sites and exposed to 21% O2 ± 100 μM DFO or to 1.5% O2 for 36 h. (E) Reconstituted cells cultured for 16 h under 21% O2 ± 100 μM DFO or to 1.5% O2, and transcription levels of pgk1 and g_lut1 were determined by quantitative real-time RT-PCR analysis.
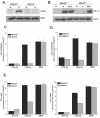
FIG. 4.
MKK3 and MKK6 are essential for hypoxic activation of HIF-1. (A) p38 MAPK activation in WT and _Mkk3/6_−/− cells exposed to 21% O2 (N) or to 1.5% O2 (H) for 30 min. (B) HIF-1α protein levels in WT and _Mkk3/6_−/− cells exposed to 21% O2 (N) ± 100 μM DFO or to 1.5% O2 (H) for 2 h. (C) WT and _Mkk3/6_−/− cells transfected with HRE-Luciferase and exposed to 21% O2 (N) ± 100 μM DFO or to 1.5% O2 (H) for 16 h. (D) WT and _Mkk3/6_−/− cells transfected with a GAL4 (1 to 147) DNA-binding domain fused to HIF-1α (531 to 826) construct and a reporter gene construct encoding five GAL4-binding sites and exposed to 21% O2 (N) ± 100 μM DFO or to 1.5% O2 (H) for 36 h (E) WT and _Mkk3/6_−/− cultured for 16 h under 21% O2 (N) ± 100 μM DFO or to 1.5% O2 (H), and transcription levels of Pgk1 and Glut1 were determined by quantitative real-time RT-PCR analysis.
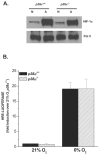
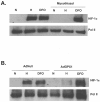
FIG. 5.
Hypoxic activation of p38 MAPK and requires mitochondrial generated oxidants. (A) ROS were determined by incubating p38α +/+ and _p38α_−/− cells with DCFH-DA (10 μM) exposed to 21% O2 ± 100 μM DFO or to 1.5% O2 ± myxothiazol, a complex III inhibitor, for 4 h. (B) p38 MAPK activation in WT cells exposed to 21% O2 (N) ± 100 μM DFO or 1.5% O2 (H) ± myxothiazol (1 μM) for 30 min. (C and D) GPX1 and p38 MAPK activation in WT cells infected with null adenovirus (control) or adenovirus expressing myc-tagged GPX1 and subsequently exposed to 21% O2 (N) ± 100 μM DFO or 1.5% O2 (H) for 2 h.
FIG. 6.
Hypoxic activation of HIF-1α protein levels requires mitochondrial generated oxidants. (A) HIF-1α protein levels in WT cells exposed to 21% O2 (N) ± 100 μM DFO or 1.5% O2 (H) ± myxothiazol (1 μM) for 2 h. (B) HIF-1α protein levels in WT cells infected with null adenovirus (control) or adenovirus expressing myc-tagged GPX1 and subsequently exposed to 21% O2 (N) ± 100 μM DFO or 1.5% O2 (H) for 2 h.
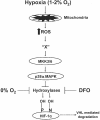
FIG. 7.
A signaling model for hypoxic activation of HIF-1. Based on our current findings we propose that hypoxia stimulates oxidant production within mitochondria. These oxidants interact with an unknown protein “X” in the cytosol to activate MKK3/6 and p38α MAPK. The activation of the p38 MAPK signaling pathway results in the decrease in hydroxylation of the HIF-1α protein at proline and asparagine residues resulting in the activation of HIF-1. By contrast, anoxia or iron chelators (DFO) during normoxia inhibit hydroxylation directly by limiting the availability of oxygen as a substrate and iron as a cofactor, respectively. Thus, anoxia or DFO does not require signaling pathways for the activation of HIF-1. Hypoxia and anoxia have distinct signaling mechanisms to activation HIF-1.
References
- Adams, R. H., A. Porras, G. Alonso, M. Jones, K. Vintersten, S. Panelli, A. Valladares, L. Perez, R. Klein, and A. R. Nebreda. 2000. Essential role of p38alpha MAP kinase in placental but not embryonic cardiovascular development. Mol. Cell 6**:**109-116. - PubMed
- Allen, M., L. Svensson, M. Roach, J. Hambor, J. McNeish, and C. A. Gabel. 2000. Deficiency of the stress kinase p38alpha results in embryonic lethality: characterization of the kinase dependence of stress responses of enzyme-deficient embryonic stem cells. J. Exp. Med. 191**:**859-870. - PMC - PubMed
- Aragones, J., D. R. Jones, S. Martin, M. A. San Juan, A. Alfranca, F. Vidal, A. Vara, I. Merida, and M. O. Landazuri. 2001. Evidence for the involvement of diacylglycerol kinase in the activation of hypoxia-inducible transcription factor 1 by low oxygen tension. J. Biol. Chem. 276**:**10548-10555. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GM60472-06/GM/NIGMS NIH HHS/United States
- P01 HL071643/HL/NHLBI NIH HHS/United States
- HL076139/HL/NHLBI NIH HHS/United States
- R01 GM060472/GM/NIGMS NIH HHS/United States
- P01HL071643-01A4/HL/NHLBI NIH HHS/United States
- T32 HL076139/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous