Importance of eIF2alpha phosphorylation and stress granule assembly in alphavirus translation regulation - PubMed (original) (raw)
Importance of eIF2alpha phosphorylation and stress granule assembly in alphavirus translation regulation
Gerald M McInerney et al. Mol Biol Cell. 2005 Aug.
Abstract
Alphavirus infection results in the shutoff of host protein synthesis in favor of viral translation. Here, we show that during Semliki Forest virus (SFV) infection, the translation inhibition is largely due to the activation of the cellular stress response via phosphorylation of eukaryotic translation initiation factor 2alpha subunit (eIF2alpha). Infection of mouse embryo fibroblasts (MEFs) expressing a nonphosphorylatable mutant of eIF2alpha does not result in efficient shutoff, despite efficient viral protein production. Furthermore, we show that the SFV translation enhancer element counteracts the translation inhibition imposed by eIF2alpha phosphorylation. In wild-type MEFs, viral infection induces the transient formation of stress granules (SGs) containing the cellular TIA-1/R proteins. These SGs are disassembled in the vicinity of viral RNA replication, synchronously with the switch from cellular to viral gene expression. We propose that phosphorylation of eIF2alpha and the consequent SG assembly is important for shutoff to occur and that the localized SG disassembly and the presence of the enhancer aid the SFV mRNAs to elude general translational arrest.
Figures
Figure 1.
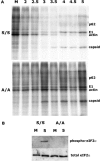
Phosphorylation of eIF2α is necessary for efficient shutoff to occur in SFV-infected cells. (A) Total protein production at various times in infected wt eIF2α (S/S) and nonphosphorylatable mutant eIF2α (A/A)-expressing MEF cells was analyzed as indicated in Materials and Methods. Positions of the cellular protein actin and viral proteins p62, E1, and capsid are indicated. All lanes are labeled in hpi or as M, mock-infected cells. (B) The phosphorylation state of eIF2α in these cells 5 h postinfection or mock infection was determined by Western blotting for phospho-eIF2α (top) and related to total eIF2α levels by stripping and reprobing the membrane (bottom).
Figure 2.
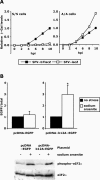
The SFV translational enhancer counteracts the translation block imposed by eIF2α phosphorylation. (A) Equal numbers of S/S and A/A cells were infected with SFV-b7lacZ or SFV-lacZ at MOI 10. Lysates were taken at different times postinfection and analyzed by an in vitro β-Gal assay. For each cell line, all protein levels are presented relative to the level of protein present in cells infected for 10 h with enhancer-containing virus. In this way, the level of translational enhancement in the cells can easily be appreciated. The results are averages of three independent experiments with similar results. (B) BHK cells were transfected with either pcDNA-EGFP or pcDNA-b12A-EGFP. Twenty-four hours later, the cells were labeled with [35S]methionine for 1 h, in the presence or absence of sodium arsenite. EGFP levels were determined by immunoprecipitation and scintillation counting. Total protein levels were determined by scintillation counting after removal of unincorporated label. For each construct, the results are presented as EGFP/total and shown relative to that value for nonstressed cells. Thus, the efficiency of each transfected gene can be related to total cellular translation in each condition. The results are averages of four independent experiments with similar results. Error bars are SD, and * indicates p < 0.05 versus nonstressed cells (Student's t test). Shown in the lower panel is Western blot analysis for phospho- and total eIF2α in each set of cells.
Figure 3.
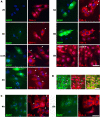
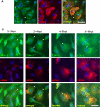
Time course of SG assembly in SFV-infected MEFs. (A) wt MEFs were either infected at low MOI with SFV-b7EGFP, mock infected, or infected with UV-inactivated SFV-b7EGFP. They were then fixed at the indicated times postinfection and processed for immunofluorescence using anti TIA-1 according to Materials and Methods. Representative images are shown for each time point. (B) eIF3 is a component of SFV-induced SGs. wt MEFs were infected at low MOI with wt SFV, fixed 5 hpi, and stained for eIF3 and TIA-1. (C) wt MEFs were infected at low MOI with SFV1-EGFP, fixed at the indicated times postinfection, and processed for immunofluorescence using anti TIA-1. Representative images are shown for each time point. Cells containing SGs are indicated with arrowheads. Bar, 50 μm.
Figure 4.
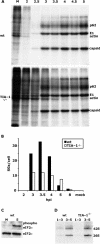
Shutoff is delayed in TIA-1-/- MEFs. (A) Total protein production at various times in wt SFV-infected wt and TIA-1-/- MEF cell lines was analyzed as indicated in Materials and Methods. Positions of the cellular protein actin and viral proteins p62, E1, and capsid are indicated. (B) The number of SGs was quantified in wt and TIA-1-/- MEFs infected with SFV-b7EFGP at low MOI. The SGs in 20 cells per time point were counted, and the mean values are plotted. (C) The phosphorylation state of eIF2α in the TIA-1-/- cells 5 h postinfection or mock infection was determined by Western blotting (top) and related to total eIF2α levels by stripping and reprobing the membrane (bottom). (D) vRNA production at the indicated times during infection of wt and TIA-1-/- MEF cell lines was analyzed as indicated in Materials and Methods. Positions of the viral genomic 42S and subgenomic 26S RNAs are indicated. All lanes are labeled in hpi or as M, mock-infected cells.
Figure 5.
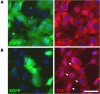
SGs in SFV-infected cells are disassembled as vRNA levels increase. (A) ISH analysis of total vRNA at 5 hpi. wt MEFs were infected at low MOI with wt SFV and fixed at 5 hpi. Hybridization and immunostaining was performed as indicated in Materials and Methods. (B) Time course of vRNA accumulation. wt MEFs were infected at low MOI with wt SFV. vRNA produced during the indicated intervals was labeled with BrUTP as indicated in Materials and Methods and processed for immunofluorescence using anti TIA-1 and anti BrUTP. Cells containing SGs are indicated with arrowheads. Bar, 50 μm.
Figure 6.
At late times postinfection, SFV-infected cells are no longer capable of arsenite-induced SG formation. wt MEFs were infected at low MOI with SFV-b7EGFP. After 8 h, cells were either fixed directly (A) or treated with sodium arsenite for 30 min and then fixed (B). Cells were processed for immunofluorescence using anti TIA-1. Cells containing SGs are indicated with arrowheads. Bar, 50 μm.
Similar articles
- RNA processing bodies are disassembled during Old World alphavirus infection.
Liu L, Weiss E, Panas MD, Götte B, Sellberg S, Thaa B, McInerney GM. Liu L, et al. J Gen Virol. 2019 Oct;100(10):1375-1389. doi: 10.1099/jgv.0.001310. J Gen Virol. 2019. PMID: 31418676 - Translational resistance of late alphavirus mRNA to eIF2alpha phosphorylation: a strategy to overcome the antiviral effect of protein kinase PKR.
Ventoso I, Sanz MA, Molina S, Berlanga JJ, Carrasco L, Esteban M. Ventoso I, et al. Genes Dev. 2006 Jan 1;20(1):87-100. doi: 10.1101/gad.357006. Genes Dev. 2006. PMID: 16391235 Free PMC article. - Mammalian orthoreovirus escape from host translational shutoff correlates with stress granule disruption and is independent of eIF2alpha phosphorylation and PKR.
Qin Q, Carroll K, Hastings C, Miller CL. Qin Q, et al. J Virol. 2011 Sep;85(17):8798-810. doi: 10.1128/JVI.01831-10. Epub 2011 Jun 29. J Virol. 2011. PMID: 21715487 Free PMC article. - The role of host eIF2α in viral infection.
Liu Y, Wang M, Cheng A, Yang Q, Wu Y, Jia R, Liu M, Zhu D, Chen S, Zhang S, Zhao XX, Huang J, Mao S, Ou X, Gao Q, Wang Y, Xu Z, Chen Z, Zhu L, Luo Q, Liu Y, Yu Y, Zhang L, Tian B, Pan L, Rehman MU, Chen X. Liu Y, et al. Virol J. 2020 Jul 23;17(1):112. doi: 10.1186/s12985-020-01362-6. Virol J. 2020. PMID: 32703221 Free PMC article. Review. - [Interaction between stress granules and viruses].
Huang Y, Hu SQ, Guo F. Huang Y, et al. Yi Chuan. 2019 Jun 20;41(6):494-508. doi: 10.16288/j.yczz.19-020. Yi Chuan. 2019. PMID: 31257198 Review. Chinese.
Cited by
- Stress granule components G3BP1 and G3BP2 play a proviral role early in Chikungunya virus replication.
Scholte FE, Tas A, Albulescu IC, Žusinaite E, Merits A, Snijder EJ, van Hemert MJ. Scholte FE, et al. J Virol. 2015 Apr;89(8):4457-69. doi: 10.1128/JVI.03612-14. Epub 2015 Feb 4. J Virol. 2015. PMID: 25653451 Free PMC article. - Superior induction of T cell responses to conserved HIV-1 regions by electroporated alphavirus replicon DNA compared to that with conventional plasmid DNA vaccine.
Knudsen ML, Mbewe-Mvula A, Rosario M, Johansson DX, Kakoulidou M, Bridgeman A, Reyes-Sandoval A, Nicosia A, Ljungberg K, Hanke T, Liljeström P. Knudsen ML, et al. J Virol. 2012 Apr;86(8):4082-90. doi: 10.1128/JVI.06535-11. Epub 2012 Feb 8. J Virol. 2012. PMID: 22318135 Free PMC article. - Chikungunya virus nsP3 blocks stress granule assembly by recruitment of G3BP into cytoplasmic foci.
Fros JJ, Domeradzka NE, Baggen J, Geertsema C, Flipse J, Vlak JM, Pijlman GP. Fros JJ, et al. J Virol. 2012 Oct;86(19):10873-9. doi: 10.1128/JVI.01506-12. Epub 2012 Jul 25. J Virol. 2012. PMID: 22837213 Free PMC article. - Humoral responses against coimmunized protein antigen but not against alphavirus-encoded antigens require alpha/beta interferon signaling.
Hidmark AS, Nordström EK, Dosenovic P, Forsell MN, Liljeström P, Karlsson Hedestam GB. Hidmark AS, et al. J Virol. 2006 Jul;80(14):7100-10. doi: 10.1128/JVI.02579-05. J Virol. 2006. PMID: 16809315 Free PMC article. - Inhibition of the ubiquitin-proteasome system induces stress granule formation.
Mazroui R, Di Marco S, Kaufman RJ, Gallouzi IE. Mazroui R, et al. Mol Biol Cell. 2007 Jul;18(7):2603-18. doi: 10.1091/mbc.e06-12-1079. Epub 2007 May 2. Mol Biol Cell. 2007. PMID: 17475769 Free PMC article.
References
- Anderson, P., and Kedersha, N. (2002). Stressful initiations. J. Cell Sci. 115, 3227-3234. - PubMed
- Barber, G. N. (2001). Host defense, viruses and apoptosis. Cell Death Differ. 8, 113-126. - PubMed
- Beattie, E., Tartaglia, J., and Paoletti, E. (1991). Vaccinia virus-encoded eIF-2 alpha homolog abrogates the antiviral effect of interferon. Virology 183, 419-422. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R56 AI033600/AI/NIAID NIH HHS/United States
- R01 AI033600/AI/NIAID NIH HHS/United States
- AI-50167/AI/NIAID NIH HHS/United States
- AI-33600/AI/NIAID NIH HHS/United States
- R01 AI050167/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources