Uterine Wnt/beta-catenin signaling is required for implantation - PubMed (original) (raw)
Comparative Study
. 2005 Jun 14;102(24):8579-84.
doi: 10.1073/pnas.0500612102. Epub 2005 Jun 1.
Affiliations
- PMID: 15930138
- PMCID: PMC1150820
- DOI: 10.1073/pnas.0500612102
Comparative Study
Uterine Wnt/beta-catenin signaling is required for implantation
Othman A Mohamed et al. Proc Natl Acad Sci U S A. 2005.
Abstract
Successful implantation relies on precisely orchestrated and reciprocal signaling between the implanting blastocyst and the receptive uterus. We have examined the role of the Wnt/beta-catenin signaling pathway during the process of implantation and demonstrate that this pathway is activated during two distinct stages. Wnt/beta-catenin signaling is first transiently activated in circular smooth muscle forming a banding pattern of activity within the uterus on early day 4. Subsequently, activation is restricted to the luminal epithelium at the prospective site of implantation. Activation at both sites requires the presence of the blastocyst. Furthermore, inhibition of Wnt/beta-catenin signaling interferes with the process of implantation. Our results demonstrate that the Wnt/beta-catenin signaling pathway plays a central role in coordinating uterus-embryo interactions required for implantation.
Figures
Fig. 1.
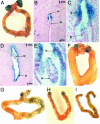
The Wnt/β-catenin signaling pathway is activated in the circular smooth muscle cells of the myometrium. (A) Whole-mount β-galactosidase staining of a uterus from a nonpregnant transgenic female. (B) Whole-mount β-galactosidase staining of a uterus from a TCF/Lef-lacZ transgenic female at mid-day 4. (C) Whole-mount β-galactosidase staining of a uterus from a TCF/Lef-lacZ transgenic female on the evening of day 4. (D and E) Sagittal (D) and transverse (E) sections of the uterus in C showing activity in the circular smooth muscle cells (csm).
Fig. 2.
Activation of the Wnt/β-catenin signaling pathway in the luminal epithelium requires the presence of the embryo and is estrogen-dependent. (A) Whole-mount β-galactosidase staining of a TCF/Lef-lacZ transgenic uterus at 1200 hours on day 5 showing restricted activity within the uterus. (B) Transverse section from a uterus at 1200 hours on day 5 showing activation of the reporter gene in the luminal epithelium (le) adjacent to the blastocyst (bl) only on the antimesometrial side (ams) of the uterus and not on the mesometrial side (ms). (C) Higher magnification of B (×50). (D) Transverse section from a TCF/Lef-lacZ uterus at 1600 hours on day 5 demonstrating that activity has spread throughout the luminal epithelium by this stage. (E) Higher magnification of D (×200). (F) Whole-mount β-galactosidase staining of a uterus from a TCF/Lef-lacZ transgenic female mated with a vasectomized male. (G) Wholemount β-galactosidase staining of a uterus from a TCF/Lef-lacZ transgenic female where the junction between the oviduct and the uterus was tightly ligated on one side. Speckled staining in uterine horn corresponds to endometrial glands. (H and I) Uterus from a pregnant TCF/Lef-lacZ transgenic female in which both ovaries were removed on the morning of day 4, before the estrogen surge, and injected with progesterone alone (H) or progesterone and estrogen (I).
Fig. 3.
Wnt proteins known to activate the canonical Wnt pathway can selectively activate Wnt/β-catenin signaling in the uterus. (A) Whole-mount β-galactosidase staining of a TCF/Lef-lacZ transgenic uterus injected with Wnt5a-conditioned media in both uterine horns. (B) Whole-mount β-galactosidase staining of a TCF/Lef-lacZ transgenic uterus injected with Wnt7a-conditioned media in both uterine horns. (C) Whole-mount β-galactosidase staining of a TCF/Lef-lacZ transgenic uterus injected with Wnt7a-conditioned media only in the right uterine horn. (D) A Cytodex-3 bead (bd) is approximately the same size as a wild-type blastocyst (bl). (E) A Cytodex-3 bead coated with Wnt7a-expressing cells. (F) Whole-mount β-galactosidase staining of a TCF/Lef-lacZ transgenic uterus injected with Wnt5a-coated Cytodex-3 beads. (G) Whole-mount β-galactosidase staining of a TCF/Lef-lacZ transgenic uterus injected with Wnt7a-coated Cytodex-3 beads.
Fig. 4.
Activation of the Wnt/β-catenin signaling pathway in the uterus is required for proper blastocyst implantation. (A) Uterine horn on day 6 in which blastocysts along with BSA had been transferred on day 4. (B) Uterine horn on day 6 in which blastocysts along with sFRP1 had been transferred on day 4. (C) Uterine horn on day 6 in which blastocysts along with sFRP2 had been transferred on day 4. (D) Uterus on day 6 in which sFRP2-conditioned media along with embryoid bodies expressing sFRP2 had been injected in the right uterine horn on the morning of day 4. The left horn was not injected and served as a control. (E) Embryos dissected from a uterine horn that had been injected with sFRP2-conditioned media along with embryoid bodies expressing sFRP2 (Right) and embryos from the same uterus from the control uterine horn (Left).
Fig. 5.
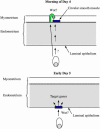
Model for blastocyst-induced Wnt/β-catenin signaling in the uterus. On the morning of day 4, the blastocyst emits a signal that results either directly or indirectly in the activation of the Wnt/β-catenin signaling pathway in discrete groups of cells within the circular smooth muscle, which form bands along the uterus. On late day 4 to early day 5, the blastocyst activates the Wnt/β-catenin signaling pathway in the luminal epithelium of the uterus. Activation of the Wnt/β-catenin signaling pathway results in the modulation of target genes in the uterus, which facilitates implantation.
Comment in
- Uterine sensing of the embryo.
Carson DD. Carson DD. Proc Natl Acad Sci U S A. 2005 Jun 14;102(24):8397-8. doi: 10.1073/pnas.0503417102. Epub 2005 Jun 6. Proc Natl Acad Sci U S A. 2005. PMID: 15939872 Free PMC article. No abstract available.
Similar articles
- Inhibition of the beta-catenin signaling pathway in blastocyst and uterus during the window of implantation in mice.
Li J, Zhang JV, Cao YJ, Zhou JX, Liu WM, Fan XJ, Duan EK. Li J, et al. Biol Reprod. 2005 Mar;72(3):700-6. doi: 10.1095/biolreprod.104.033837. Epub 2004 Oct 20. Biol Reprod. 2005. PMID: 15496516 - Embryo-uterine cross-talk during implantation: the role of Wnt signaling.
Chen Q, Zhang Y, Lu J, Wang Q, Wang S, Cao Y, Wang H, Duan E. Chen Q, et al. Mol Hum Reprod. 2009 Apr;15(4):215-21. doi: 10.1093/molehr/gap009. Epub 2009 Feb 17. Mol Hum Reprod. 2009. PMID: 19223336 Review. - Cellular localization and signaling activity of beta-catenin in migrating neural crest cells.
de Melker AA, Desban N, Duband JL. de Melker AA, et al. Dev Dyn. 2004 Aug;230(4):708-26. doi: 10.1002/dvdy.20091. Dev Dyn. 2004. PMID: 15254905 - Uterine sensing of the embryo.
Carson DD. Carson DD. Proc Natl Acad Sci U S A. 2005 Jun 14;102(24):8397-8. doi: 10.1073/pnas.0503417102. Epub 2005 Jun 6. Proc Natl Acad Sci U S A. 2005. PMID: 15939872 Free PMC article. No abstract available. - Signal transduction during amyloid-beta-peptide neurotoxicity: role in Alzheimer disease.
Fuentealba RA, Farias G, Scheu J, Bronfman M, Marzolo MP, Inestrosa NC. Fuentealba RA, et al. Brain Res Brain Res Rev. 2004 Dec;47(1-3):275-89. doi: 10.1016/j.brainresrev.2004.07.018. Brain Res Brain Res Rev. 2004. PMID: 15572177 Review.
Cited by
- A historical review of blastocyst implantation research.
Yoshinaga K. Yoshinaga K. Biol Reprod. 2018 Jul 1;99(1):175-195. doi: 10.1093/biolre/ioy093. Biol Reprod. 2018. PMID: 30010858 Free PMC article. Review. - The Mechanism of Wnt Pathway Regulated by Telocytes to Promote the Regeneration and Repair of Intrauterine Adhesions.
Zhao CZ, Fu P, Peng WY, Pan N, Peng Y, Zhao M, Xie W. Zhao CZ, et al. Comput Math Methods Med. 2022 Jul 6;2022:3809792. doi: 10.1155/2022/3809792. eCollection 2022. Comput Math Methods Med. 2022. PMID: 35844454 Free PMC article. Retracted. - The androgen and progesterone receptors regulate distinct gene networks and cellular functions in decidualizing endometrium.
Cloke B, Huhtinen K, Fusi L, Kajihara T, Yliheikkilä M, Ho KK, Teklenburg G, Lavery S, Jones MC, Trew G, Kim JJ, Lam EW, Cartwright JE, Poutanen M, Brosens JJ. Cloke B, et al. Endocrinology. 2008 Sep;149(9):4462-74. doi: 10.1210/en.2008-0356. Epub 2008 May 29. Endocrinology. 2008. PMID: 18511503 Free PMC article. - Characterization of the role for cadherin 6 in the regulation of human endometrial receptivity.
Zhou W, Santos L, Dimitriadis E. Zhou W, et al. Reprod Biol Endocrinol. 2020 Jun 29;18(1):66. doi: 10.1186/s12958-020-00624-w. Reprod Biol Endocrinol. 2020. PMID: 32600462 Free PMC article. - Evolution of Embryo Implantation Was Enabled by the Origin of Decidual Stromal Cells in Eutherian Mammals.
Chavan AR, Griffith OW, Stadtmauer DJ, Maziarz J, Pavlicev M, Fishman R, Koren L, Romero R, Wagner GP. Chavan AR, et al. Mol Biol Evol. 2021 Mar 9;38(3):1060-1074. doi: 10.1093/molbev/msaa274. Mol Biol Evol. 2021. PMID: 33185661 Free PMC article.
References
- Paria, B. C., Reese, J., Das, S. K. & Dey, S. K. (2002) Science 296, 2185-2188. - PubMed
- Das, S. K., Wang, X. N., Paria, B. C., Damm, D., Abraham, J. A., Klagsbrun, M., Andrews, G. K. & Dey, S. K. (1994) Development (Cambridge, U.K.) 120, 1071-1083. - PubMed
- Das, S. K., Das, N., Wang, J., Lim, H., Schryver, B., Plowman, G. D. & Dey, S. K. (1997) Dev. Biol. 190, 178-190. - PubMed
- Dey, S. K., Lim, H., Das, S. K., Reese, J., Paria, B. C., Daikoku, T. & Wang, H. (2004) Endocr. Rev. 25, 341-373. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources