Electrolytic lesions of the dorsal hippocampus disrupt renewal of conditional fear after extinction - PubMed (original) (raw)
Electrolytic lesions of the dorsal hippocampus disrupt renewal of conditional fear after extinction
Jinzhao Ji et al. Learn Mem. 2005 May-Jun.
Abstract
There is a growing body of evidence that the hippocampus is critical for context-dependent memory retrieval. In the present study, we used Pavlovian fear conditioning in rats to examine the role of the dorsal hippocampus (DH) in the context-specific expression of fear memory after extinction (i.e., renewal). Pre-training electrolytic lesions of the DH blunted the expression of conditional freezing to an auditory conditional stimulus (CS), but did not affect the acquisition of extinction to that CS. In contrast, DH lesions impaired the context-specific expression of extinction, eliminating the renewal of fear normally observed to a CS presented outside of the extinction context. Post-extinction DH lesions also eliminated the context dependence of fear extinction. These results are consistent with those using pharmacological inactivation of the DH and suggest that the DH is required for using contextual stimuli to regulate the expression of fear to a Pavlovian CS after extinction.
Figures
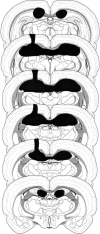
Figure 1.
Schematic representation of a representative electrolytic dorsal hippocampal lesion. Reconstruction of the lesion was made on rat atlas templates adapted with permission from Elsevier © 1992, Swanson (1992).
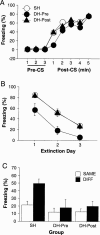
Figure 2.
Effects of DH lesions on ABB/AAB renewal. (A) Mean (±SEM) percentage of freezing exhibited on the conditioning day, with 3 min prior to tone CS onset (baseline) and 1 min post-CS for five trials. All rats were fear conditioned in context A. (B) Extinction to the tone CS. Mean (±SEM) percentage of freezing for the first four CS presentations across the 3 d of extinction in contexts A and B. (C) Mean (±SEM) percentage of freezing for the first 4 min after CS onset during test. Rats were tested for fear of CS in a neutral context, either in the extinction context (SAME; ABB; open bars) or outside of the extinction context (DIFF; AAB; filled bars). Electrolytic DH or sham (SH) lesions were made either before training (DH-Pre) or after extinction (DH-Post).
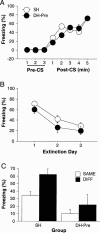
Figure 3.
Effects of DH lesions on AAA/ABA renewal. (A) Mean (±SEM) percentage of freezing exhibited on the conditioning day, with 3 min prior to tone CS onset (baseline) and 1 min post-CS for five trials. All rats were fear conditioned in context A. (B) Extinction to the tone CS. Mean (±SEM) percentage of freezing for the first four CS presentations across the 3 d of extinction in contexts A and B. (C) Mean (±SEM) percentage of freezing for the first 4 min after CS onset during test. Rats were tested for fear of CS in the conditioning context, either in the extinction context (SAME; AAA; open bars) or outside of the extinction context (DIFF; ABA; filled bars). Electrolytic DH (DH-Pre) or sham (SH) lesions were made before conditioning.
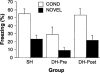
Figure 4.
Effects of DH lesions on context discrimination. Mean (±SEM) percentage of freezing for the first 8 min of context exposure on the first equilibration day. All rats were fear conditioned in context A. Exposure was either in the same context as the conditioning context (COND, open bars) or in a novel context (NOVEL, filled bars). Electrolytic DH or sham (SH) lesions were made either before training (DH-Pre) or after extinction (DH-Post).
Similar articles
- Factors regulating the effects of hippocampal inactivation on renewal of conditional fear after extinction.
Corcoran KA, Maren S. Corcoran KA, et al. Learn Mem. 2004 Sep-Oct;11(5):598-603. doi: 10.1101/lm.78704. Learn Mem. 2004. PMID: 15466314 Free PMC article. - Hippocampal inactivation disrupts contextual retrieval of fear memory after extinction.
Corcoran KA, Maren S. Corcoran KA, et al. J Neurosci. 2001 Mar 1;21(5):1720-6. doi: 10.1523/JNEUROSCI.21-05-01720.2001. J Neurosci. 2001. PMID: 11222661 Free PMC article. - Intact renewal after extinction of conditioned suppression with lesions of either the retrosplenial cortex or dorsal hippocampus.
Todd TP, Jiang MY, DeAngeli NE, Bucci DJ. Todd TP, et al. Behav Brain Res. 2017 Mar 1;320:143-153. doi: 10.1016/j.bbr.2016.11.033. Epub 2016 Nov 21. Behav Brain Res. 2017. PMID: 27884768 Free PMC article. - Hippocampal involvement in contextual modulation of fear extinction.
Ji J, Maren S. Ji J, et al. Hippocampus. 2007;17(9):749-58. doi: 10.1002/hipo.20331. Hippocampus. 2007. PMID: 17604353 Review. - Is the hippocampus necessary for contextual fear conditioning?
Gewirtz JC, McNish KA, Davis M. Gewirtz JC, et al. Behav Brain Res. 2000 Jun 1;110(1-2):83-95. doi: 10.1016/s0166-4328(99)00187-4. Behav Brain Res. 2000. PMID: 10802306 Review.
Cited by
- The Plasticity of Extinction: Contribution of the Prefrontal Cortex in Treating Addiction through Inhibitory Learning.
Gass JT, Chandler LJ. Gass JT, et al. Front Psychiatry. 2013 May 30;4:46. doi: 10.3389/fpsyt.2013.00046. eCollection 2013. Front Psychiatry. 2013. PMID: 23750137 Free PMC article. - Transgenic inhibition of neuronal protein kinase A activity facilitates fear extinction.
Isiegas C, Park A, Kandel ER, Abel T, Lattal KM. Isiegas C, et al. J Neurosci. 2006 Dec 6;26(49):12700-7. doi: 10.1523/JNEUROSCI.2743-06.2006. J Neurosci. 2006. PMID: 17151273 Free PMC article. - Differential effects of controllable and uncontrollable footshock stress on sleep in mice.
Sanford LD, Yang L, Wellman LL, Liu X, Tang X. Sanford LD, et al. Sleep. 2010 May;33(5):621-30. doi: 10.1093/sleep/33.5.621. Sleep. 2010. PMID: 20469804 Free PMC article. - Double dissociation between the anterior cingulate cortex and nucleus accumbens core in encoding the context versus the content of pavlovian cocaine cue extinction.
Torregrossa MM, Gordon J, Taylor JR. Torregrossa MM, et al. J Neurosci. 2013 May 8;33(19):8370-7. doi: 10.1523/JNEUROSCI.0489-13.2013. J Neurosci. 2013. PMID: 23658176 Free PMC article. - Comparison of behavioral and brain indices of fear renewal during a standard vs. novel immersive reality Pavlovian fear extinction paradigm in healthy adults.
Zabik NL, Peters C, Iadipaolo A, Marusak HA, Rabinak CA. Zabik NL, et al. Behav Brain Res. 2023 Feb 2;437:114154. doi: 10.1016/j.bbr.2022.114154. Epub 2022 Oct 13. Behav Brain Res. 2023. PMID: 36244544 Free PMC article.
References
- Addis, D.R., Moscovitch, M., Crawley, A.P., and McAndrews, M.P. 2004. Recollective qualities modulate hippocampal activation during autobiographical memory retrieval. Hippocampus. 14: 752-762. - PubMed
- Bouton, M.E. 1993. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychol. Bull. 114: 80-99. - PubMed
- Burgess, N., Maguire, E.A., Spiers, H.J., and O'Keefe, J. 2001. A temporoparietal and prefrontal network for retrieving the spatial context of lifelike events. Neuroimage 14: 439-453. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources