AMPA/kainate, NMDA, and dopamine D1 receptor function in the nucleus accumbens core: a context-limited role in the encoding and consolidation of instrumental memory - PubMed (original) (raw)
AMPA/kainate, NMDA, and dopamine D1 receptor function in the nucleus accumbens core: a context-limited role in the encoding and consolidation of instrumental memory
Pepe J Hernandez et al. Learn Mem. 2005 May-Jun.
Abstract
Neural integration of glutamate- and dopamine-coded signals within the nucleus accumbens (NAc) is a fundamental process governing cellular plasticity underlying reward-related learning. Intra-NAc core blockade of NMDA or D1 receptors in rats impairs instrumental learning (lever-pressing for sugar pellets), but it is not known during which phase of learning (acquisition or consolidation) these receptors are recruited, nor is it known what role AMPA/kainate receptors have in these processes. Here we show that pre-trial intra-NAc core administration of the NMDA, AMPA/KA, and D1 receptor antagonists AP-5 (1 microg/0.5 microL), LY293558 (0.01 or 0.1 microg/0.5 microL), and SCH23390 (1 microg/0.5 microL), respectively, impaired acquisition of a lever-pressing response, whereas post-trial administration left memory consolidation unaffected. An analysis of the microstructure of behavior while rats were under the influence of these drugs revealed that glutamatergic and dopaminergic signals contribute differentially to critical aspects of the initial, randomly emitted behaviors that enable reinforcement learning. Thus, glutamate and dopamine receptors are activated in a time-limited fashion-only being required while the animals are actively engaged in the learning context.
Figures
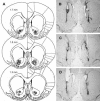
Figure 1.
Histological analyses of representative nucleus accumbens core injections' sites. (A) Stereotaxic coordinates displayed within the sections are in millimeters from bregma. NAc core injection sites representative of LY293558, AP-5, and SCH23390 infusions are indicated by circles, triangles, and squares, respectively. Adapted with permission from Elsevier © 1998, Paxinos and Watson (1998). (_B_-D) Nissl stains of coronal sections indicating cannulae and injector tracts terminating in the NAc core for the LY293558, AP-5, and SCH23390 experiments, respectively.
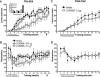
Figure 2.
Memory consolidation is not affected by AMPA/KA receptor blockade. (A,B) Pre-trial infusions of LY293558 (0, 0.01, or 0.1 μg) dose-dependently impaired the acquisition of instrumental learning as measured by decreased (A) lever presses and (B) nose pokes. Infusions after the task was learned had no effect on memory retrieval/performance. (Inset) Lever presses made over the first three sessions. Vehicle group, n = 6; 0.01 μg group, n = 5; 0.1 μg group, n =6. (C,D) Post-trial infusions of LY293558 (0 or 0.1 μg) failed to affect (C) lever-pressing or (D) nose-poking, indicating memory for the task was consolidated normally. Vehicle group, n = 7; 0.1 μg group, n = 5. Arrows next to session 3 in B and D mark the end of noncontingent reinforcement. For all panels, data are shown as the mean number of lever presses or nose pokes ± SEM. Brackets and arrows beneath the _x_-axis indicate the frequency of injections. (**) Main effect of treatment on lever-pressing, P < 0.01 (see Results for nose-poke statistics).
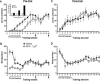
Figure 3.
AMPA/KA receptor blockade disrupts specific patterns of behavior during operant training. (_A_-D) An analysis of the microstructure of behavior of rats given pre-trial infusions of LY293558 (0, 0.01, or 0.1 μg) (see Fig. 2A,B). The 0.1-μg dose of LY293558 prevented (A) the learning-related decrease in the probability to make consecutive nose pokes, Pr(NP|NP), and (B) the increase in the probability to lever-press after retrieving a reward, Pr(LP|NP), as demonstrated by controls and the 0.01-μg group, until the task was well-learned. No reliable changes were observed in (C) the probability to retrieve a reward upon delivery, Pr(NP|Reinf), or (D) the latency to retrieve rewards. Post-learning infusions had no effect. Error bars indicate the SEM. Brackets and arrows beneath the _x_-axis indicate the frequency of pre-trial injections.
Figure 4.
NMDA receptor activity is not necessary for memory consolidation. (A,B) Pre-trial infusions of AP-5 (0 or 1 μg) impaired the acquisition of instrumental learning as measured by decreased (A) lever presses and (B) nose pokes. Infusions after the task was consolidated show no effect on memory retrieval or performance. (Inset) Lever presses made over the first three sessions. Vehicle group, n =7; 1 μg group, n =8. (C,D) Post-trial infusions of AP-5 (0 or 1 μg) failed to affect (C) lever-pressing or (D) nose-poking, indicating memory for the task was consolidated normally. Vehicle group, n = 7; 1 μg group, n = 8. Arrows next to session 3 in B and D mark the end of noncontingent reinforcement. For all panels, data are shown as the mean number of lever presses or nose pokes ± SEM. Brackets and arrows beneath the _x_-axis indicate the frequency of injections. (**) Main effect of treatment on lever-pressing in the pre-trial condition, P < 0.01 (see Results for nose-poke statistics).
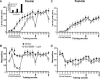
Figure 5.
NMDA receptor blockade differentially disrupts specific patterns of behavior during operant training. (_A_-D) An analysis of the microstructure of behavior of rats given pre-trial infusions of AP-5 (0 or 1 μg) (see Fig. 4A,B). (A) Unlike LY293558, AP-5 decreased the tendency to make consecutive nose pokes, Pr(NP|NP), while free rewards were given (see Materials and Methods), then increased the Pr(NP|NP) relative to controls until the task was well-learned. (B) AP-5 reduced the normal increase in the probability to lever-press after retrieving a reward and, in contrast to LY293558, AP-5 also (C) reduced the probability to retrieve rewards while (D) increasing the latency in which to retrieve them until the task was well-learned. Post-learning infusions had no effect. Error bars indicate the SEM. Brackets and arrows beneath the _x_-axis indicate the frequency of pre-trial injections.
Figure 6.
D1 receptor blockade does not affect memory consolidation. Pre-trial infusions of SCH23390 (0 or 1 μg) impaired the acquisition of instrumental learning as measured by decreased (A) lever presses and (B) nose pokes. Only infusions of SCH29330 after the task was consolidated significantly decreased lever-pressing, whereas a nonsignificant decrease in nose-poking was observed. (Inset) Lever presses made over the first three sessions. Vehicle group, n = 9; 1 μg group, n =8. Post-trial infusions of SCH23390 (0 or 1 μg) failed to affect (C) lever-pressing or (D) nose-poking, indicating that memory for the task was consolidated normally. Vehicle group, n = 8; 1 μg group, n = 7. Arrows next to session 3 in B and D mark the end of noncontingent reinforcement. For all panels, data are shown as the mean number of lever presses or nose pokes ± SEM. Brackets and arrows beneath the _x_-axis indicate the frequency of injections. (*) Main effect of treatment over sessions 1-10 on lever-pressing, P ≤ 0.05. (†) Main effect of treatment over sessions 10-11 on lever-pressing, P ≤ 0.05 during the pre-trial condition (see Results for nose-poke statistics).
Figure 7.
Antagonism of D1 receptors produces disruptions in the microstructure of behavior similar to those caused by LY293558. (_A_-D) An analysis of the microstructure of behavior of rats given pre-trial infusions of SCH23390 (0 or 1 μg) (see Fig. 6A,B). Until the task was well learned, the 1-μg dose of SCH23390, like LY293558, prevented (A) the learning-related decrease in the probability to make consecutive nose pokes, Pr(NP|NP), and (B) the increase in the probability to lever-press after retrieving a reward, Pr(LP|NP), as demonstrated by controls. No reliable changes were observed in (C) the probability to retrieve a reward upon delivery, Pr(NP|Reinf), or (D) the latency to retrieve rewards. Post-learning infusions had no apparent effect. Error bars indicate the SEM. Brackets and arrows beneath the _x_-axis indicate the frequency of pre-trial injections.
Similar articles
- Roles of hippocampal NMDA receptors and nucleus accumbens D1 receptors in the amphetamine-produced conditioned place preference in rats.
Tan SE. Tan SE. Brain Res Bull. 2008 Dec 16;77(6):412-9. doi: 10.1016/j.brainresbull.2008.09.007. Epub 2008 Oct 16. Brain Res Bull. 2008. PMID: 18929625 - Differential involvement of NMDA, AMPA/kainate, and dopamine receptors in the nucleus accumbens core in the acquisition and performance of pavlovian approach behavior.
Di Ciano P, Cardinal RN, Cowell RA, Little SJ, Everitt BJ. Di Ciano P, et al. J Neurosci. 2001 Dec 1;21(23):9471-7. doi: 10.1523/JNEUROSCI.21-23-09471.2001. J Neurosci. 2001. PMID: 11717381 Free PMC article. - Involvement of NMDA and AMPA/KA receptors in the nucleus accumbens core in instrumental learning guided by reward-predictive cues.
Giertler C, Bohn I, Hauber W. Giertler C, et al. Eur J Neurosci. 2005 Mar;21(6):1689-702. doi: 10.1111/j.1460-9568.2005.03983.x. Eur J Neurosci. 2005. PMID: 15845096 - Glutamate-mediated plasticity in corticostriatal networks: role in adaptive motor learning.
Kelley AE, Andrzejewski ME, Baldwin AE, Hernandez PJ, Pratt WE. Kelley AE, et al. Ann N Y Acad Sci. 2003 Nov;1003:159-68. doi: 10.1196/annals.1300.061. Ann N Y Acad Sci. 2003. PMID: 14684443 Review. - Targeting the glutamatergic system to counteract organophosphate poisoning: A novel therapeutic strategy.
Aroniadou-Anderjaska V, Figueiredo TH, Apland JP, Braga MF. Aroniadou-Anderjaska V, et al. Neurobiol Dis. 2020 Jan;133:104406. doi: 10.1016/j.nbd.2019.02.017. Epub 2019 Feb 21. Neurobiol Dis. 2020. PMID: 30798006 Review.
Cited by
- A "good parent" function of dopamine: transient modulation of learning and performance during early stages of training.
Horvitz JC, Choi WY, Morvan C, Eyny Y, Balsam PD. Horvitz JC, et al. Ann N Y Acad Sci. 2007 May;1104:270-88. doi: 10.1196/annals.1390.017. Epub 2007 Mar 14. Ann N Y Acad Sci. 2007. PMID: 17360799 Free PMC article. Review. - Acute and chronic dopamine receptor stimulation modulates AMPA receptor trafficking in nucleus accumbens neurons cocultured with prefrontal cortex neurons.
Sun X, Milovanovic M, Zhao Y, Wolf ME. Sun X, et al. J Neurosci. 2008 Apr 16;28(16):4216-30. doi: 10.1523/JNEUROSCI.0258-08.2008. J Neurosci. 2008. PMID: 18417701 Free PMC article. - HIV-1 Tat regulation of dopamine transmission and microglial reactivity is brain region specific.
Miller DR, Shaerzadeh F, Phan L, Sharif N, Gamble-George J, McLaughlin JP, Streit WJ, Khoshbouei H. Miller DR, et al. Glia. 2018 Sep;66(9):1915-1928. doi: 10.1002/glia.23447. Epub 2018 May 7. Glia. 2018. PMID: 29733459 Free PMC article. - Critical role of NMDA but not opioid receptors in the acquisition of fat-conditioned flavor preferences in rats.
Dela Cruz JA, Bae VS, Icaza-Cukali D, Sampson C, Bamshad D, Samra A, Singh S, Khalifa N, Touzani K, Sclafani A, Bodnar RJ. Dela Cruz JA, et al. Neurobiol Learn Mem. 2012 Nov;98(4):341-7. doi: 10.1016/j.nlm.2012.10.007. Epub 2012 Oct 24. Neurobiol Learn Mem. 2012. PMID: 23103774 Free PMC article. - An NMDA antagonist in the MPOA impairs copulation and stimulus sensitization in male rats.
Vigdorchik AV, Parrish BP, Lagoda GA, McHenry JA, Hull EM. Vigdorchik AV, et al. Behav Neurosci. 2012 Feb;126(1):186-95. doi: 10.1037/a0026460. Behav Neurosci. 2012. PMID: 22289046 Free PMC article.
References
- Alexander, G.E., DeLong, M.R., and Strick, P.L. 1986. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Ann. Rev. Neurosci. 9: 357-381. - PubMed
- Bakshi, V.P. and Kelley, A.E. 1991. Dopaminergic regulation of feeding behavior: Differential effects of haloperidol infusion into three striatal subregions. Psychobiology 19: 223-232.
- Baldo, B.A., Sadeghian, K., Basso, A.M., and Kelley, A.E. 2002. Effects of selective dopamine D1 or D2 receptor blockade within nucleus accumbens subregions on ingestive behavior and associated motor activity. Behav. Brain Res. 137: 165-177. - PubMed
- Baldwin, A.E., Sadeghian, K., Holahan, M.R., and Kelley, A.E. 2002a. Appetitive instrumental learning is impaired by inhibition of cAMP-dependent protein kinase within the nucleus accumbens. Neurobiol. Learn. Mem. 77: 44-62. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical