Host control of Mycobacterium tuberculosis is regulated by 5-lipoxygenase-dependent lipoxin production - PubMed (original) (raw)
Comparative Study
. 2005 Jun;115(6):1601-6.
doi: 10.1172/JCI23949.
Affiliations
- PMID: 15931391
- PMCID: PMC1136995
- DOI: 10.1172/JCI23949
Comparative Study
Host control of Mycobacterium tuberculosis is regulated by 5-lipoxygenase-dependent lipoxin production
Andre Bafica et al. J Clin Invest. 2005 Jun.
Abstract
Th1 type cytokine responses are critical in the control of Mycobacterium tuberculosis infection. Recent findings indicate that 5-lipoxygenase-dependent (5-LO-dependent) lipoxins regulate host IL-12 production in vivo. Here, we establish lipoxins as key chemical mediators in resistance to M. tuberculosis infection. High levels of lipoxin A4 (LXA4) were detected in sera from infected WT but not infected 5-LO-deficient mice. Moreover, lungs from M. tuberculosis-infected 5-lo-/- animals showed increased IL-12, IFN-gamma, and NO synthase 2 (NOS2) mRNA levels compared with the same tissues in WT mice. Similarly, splenocyte recall responses were enhanced in mycobacteria-infected 5-lo-/- versus WT mice. Importantly, bacterial burdens in 5-lo-/- lungs were significantly lower than those from WT mice, and this enhancement in the resistance of the 5-lo-/- animals to M. tuberculosis was completely prevented by administration of a stable LXA4 analog. Together our results demonstrate that lipoxins negatively regulate protective Th1 responses against mycobacterial infection in vivo and suggest that the inhibition of lipoxin biosynthesis could serve as a strategy for enhancing host resistance to M. tuberculosis.
Figures
Figure 1
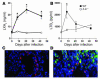
5-LO–dependent LXA4 and LTB4 production and 5-LO expression during M. tuberculosis infection. WT (B6, 129J F2; filled squares) and 5-LO–deficient (B6, 129J Alox-5; open circles) animals were infected by aerosol exposure with an average of 300 CFU/mouse of M. tuberculosis H37Rv and LXA4 (A) and LTB4 (B) assessed by ELISA in serum at 8, 21 and 42 days after infection. Results are mean ± SE of measurements from 5 animals. *P < 0.05 between experimental groups. Results shown are representative of 2 independent experiments. (C and D) WT lung sections were stained with anti–5-LO (red) and costained with anti-F4/80 (green), followed by counterstaining with DAPI (blue). (C) 5-LO+ endothelium. (D) Several F4/80+5-LO+ cells infiltrating pulmonary tissue during M. tuberculosis infection. Original magnification, ×63.
Figure 2
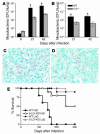
Increased resistance of 5-LO–deficient mice to M. tuberculosis infection. Lungs from WT (black bars) and 5-lo–/– (gray bars) mice were harvested at several time points after infection with an average of 300 (A) or 50 (B) CFU/mouse and mycobacterial burdens determined. Results are mean ± SE of measurements from 4 animals. *P < 0.05. Representative acid-fast bacilli–stained sections from lungs of 50-day–infected WT (C) and 5-lo–/– (D) mice (300 CFU/animal) illustrate the reduction in acid-fast bacilli (red staining) in the KO animals. Original magnification, ×63. (E) WT B6, 129S F2/J (filled symbols) and 5-LO–deficient (open symbols) animals were aerogenically infected with an average of 300 CFU/mouse (high dose [HiD]; squares) or with 50 CFU/mouse (low dose [LoD]; circles) (n = 10 animals per group) and survival monitored. The results shown are representative of 2 independent experiments performed at each dose.
Figure 3
Decreased inflammation in lungs of M. tuberculosis–infected 5-LO–deficient mice. Representative H&E-stained sections of lungs from 50-day–infected (300 CFU inoculum) B6, 129S F2/J control (A and B) and 5-LO–deficient (C and D) animals. Note the reduction in inflammatory infiltration and greatly increased alveolar space in 5-lo–/– animals (C and D). Original magnification, ×5 (A and C) and ×40 (B and D).
Figure 4
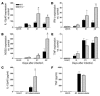
5-LO–deficient mice infected with M. tuberculosis display increased expression of proinflammatory mediators. WT and 5-lo–/– mice were aerogenically infected (300 CFU inoculum), and relative expression of mRNAs for IL-12p40 (A), NOS2 (B), IFN-γ (D), and TNF (E) was determined in the lungs at 8, 21 and 42 days after M. tuberculosis infection. To further confirm these observations, we prepared lung homogenates from the same animal groups shown above and determined IL-12 (C) and TNF (F) levels by ELISA. *P < 0.05. The results shown are representative of 2 independent experiments. Uninf., uninfected.
Figure 5
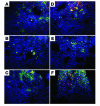
Lung-infiltrating DCs and macrophages express high levels of IL-12 and NOS2 in 5-LO–deficient hosts infected with M. tuberculosis. Frozen sections of lungs from infected (300 CFU inoculum) WT (A, B, and C) and 5-lo–/– (D, E, and F) mice were double stained with anti-CD11c (A and D) or anti-F4/80 (B, C, E, and F) (green) and with anti–IL-12p40 (A and D), anti-TNF (B and E), or anti-NOS2 (C and F) (red), then counterstained with DAPI (blue). Note the presence of many more CD11c+IL-12p40+ cells and F4/80+NOS2+ cells in the tissue sections from 5-lo–/– animals. The asterisk in A indicates the presence of multinucleated cells at the center of a granuloma. Representative micrographs (magnification, ×63) from 4 animals per group are shown.
Figure 6
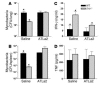
In vivo administration of a stable analog of LXA4 in M. tuberculosis–infected mice. WT or 5-lo–/– mice were infected by aerosol exposure with M. tuberculosis (300 CFU inoculum) and treated 3 times a week by gavage from days 2 to 20 with vehicle or ATLa2 at 100 ng/animal/treatment. Mice were sacrificed, and mycobacterial burdens were assessed in lungs (A) and spleens (B), 21 days after infection. Spleen cell cultures from the same animals were restimulated with purified protein derivative, and 72 hours later, IFN-γ (C) and TNF (D) levels were measured in supernatants by ELISA. Results are the mean ± SD from 5 animals per group. *P < 0.05 between groups.
Comment in
- An oily, sustained counter-regulatory response to TB.
Karp CL, Cooper AM. Karp CL, et al. J Clin Invest. 2005 Jun;115(6):1473-6. doi: 10.1172/JCI25353. J Clin Invest. 2005. PMID: 15931386 Free PMC article.
Similar articles
- 5-Lipoxygenase negatively regulates Th1 response during Brucella abortus infection in mice.
Fahel JS, de Souza MB, Gomes MT, Corsetti PP, Carvalho NB, Marinho FA, de Almeida LA, Caliari MV, Machado FS, Oliveira SC. Fahel JS, et al. Infect Immun. 2015 Mar;83(3):1210-6. doi: 10.1128/IAI.02592-14. Epub 2015 Jan 12. Infect Immun. 2015. PMID: 25583526 Free PMC article. - Inhibition of leukotriene biosynthesis abrogates the host control of Mycobacterium tuberculosis.
Peres CM, de Paula L, Medeiros AI, Sorgi CA, Soares EG, Carlos D, Peters-Golden M, Silva CL, Faccioli LH. Peres CM, et al. Microbes Infect. 2007 Apr;9(4):483-9. doi: 10.1016/j.micinf.2007.01.006. Epub 2007 Jan 21. Microbes Infect. 2007. PMID: 17347013 Free PMC article. - Lower expression of Th1-related cytokines and inducible nitric oxide synthase in mice with streptozotocin-induced diabetes mellitus infected with Mycobacterium tuberculosis.
Yamashiro S, Kawakami K, Uezu K, Kinjo T, Miyagi K, Nakamura K, Saito A. Yamashiro S, et al. Clin Exp Immunol. 2005 Jan;139(1):57-64. doi: 10.1111/j.1365-2249.2005.02677.x. Clin Exp Immunol. 2005. PMID: 15606614 Free PMC article. - Immunity to tuberculosis.
North RJ, Jung YJ. North RJ, et al. Annu Rev Immunol. 2004;22:599-623. doi: 10.1146/annurev.immunol.22.012703.104635. Annu Rev Immunol. 2004. PMID: 15032590 Review. - TB: the Yin and Yang of lipid mediators.
Tobin DM, Ramakrishnan L. Tobin DM, et al. Curr Opin Pharmacol. 2013 Aug;13(4):641-5. doi: 10.1016/j.coph.2013.06.007. Epub 2013 Jul 9. Curr Opin Pharmacol. 2013. PMID: 23849093 Free PMC article. Review.
Cited by
- Host-Directed Therapies for Tuberculosis.
Jeong EK, Lee HJ, Jung YJ. Jeong EK, et al. Pathogens. 2022 Nov 3;11(11):1291. doi: 10.3390/pathogens11111291. Pathogens. 2022. PMID: 36365041 Free PMC article. Review. - The Interplay Between Systemic Inflammation, Oxidative Stress, and Tissue Remodeling in Tuberculosis.
Amaral EP, Vinhaes CL, Oliveira-de-Souza D, Nogueira B, Akrami KM, Andrade BB. Amaral EP, et al. Antioxid Redox Signal. 2021 Feb 20;34(6):471-485. doi: 10.1089/ars.2020.8124. Epub 2020 Jun 19. Antioxid Redox Signal. 2021. PMID: 32559410 Free PMC article. Review. - ALOX5 is associated with tuberculosis in a subset of the pediatric population of North China.
Shen C, Wu XR, Wang BB, Sun L, Jiao WW, Wang J, Feng WX, Xiao J, Miao Q, Liu F, Yin QQ, Ma X, Shen AD. Shen C, et al. Genet Test Mol Biomarkers. 2013 Apr;17(4):284-8. doi: 10.1089/gtmb.2012.0426. Epub 2013 Feb 28. Genet Test Mol Biomarkers. 2013. PMID: 23448388 Free PMC article. - Narrative Review of _n_-3 Polyunsaturated Fatty Acid Supplementation upon Immune Functions, Resolution Molecules and Lipid Peroxidation.
Zaloga GP. Zaloga GP. Nutrients. 2021 Feb 18;13(2):662. doi: 10.3390/nu13020662. Nutrients. 2021. PMID: 33670710 Free PMC article. Review. - Mycobacterium tuberculosis evades macrophage defenses by inhibiting plasma membrane repair.
Divangahi M, Chen M, Gan H, Desjardins D, Hickman TT, Lee DM, Fortune S, Behar SM, Remold HG. Divangahi M, et al. Nat Immunol. 2009 Aug;10(8):899-906. doi: 10.1038/ni.1758. Epub 2009 Jun 28. Nat Immunol. 2009. PMID: 19561612 Free PMC article.
References
- Flynn JL, et al. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561–572. - PubMed
- Flynn JL, et al. IL-12 increases resistance of BALB/c mice to Mycobacterium tuberculosis infection. J. Immunol. 1995;155:2515–2524. - PubMed
- de Jong R, et al. Severe mycobacterial and Salmonella infections in interleukin-12 receptor-deficient patients. Science. 1998;280:1435–1438. - PubMed
- Altare F, et al. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science. 1998;280:1432–1435. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases