Hydrogen sulfide causes vanilloid receptor 1-mediated neurogenic inflammation in the airways - PubMed (original) (raw)
Hydrogen sulfide causes vanilloid receptor 1-mediated neurogenic inflammation in the airways
Marcello Trevisani et al. Br J Pharmacol. 2005 Aug.
Abstract
Hydrogen sulfide (H(2)S) is described as a mediator of diverse biological effects, and is known to produce irritation and injury in the lung following inhalation. Recently, H(2)S has been found to cause contraction in the rat urinary bladder via a neurogenic mechanism. Here, we studied whether sodium hydrogen sulfide (NaHS), used as donor of H(2)S, produces responses mediated by sensory nerve activation in the guinea-pig airways. NaHS evoked an increase in neuropeptide release in the airways that was significantly attenuated by capsaicin desensitization and by the transient receptor potential vanilloid 1 (TRPV1) antagonist capsazepine. In addition, NaHS caused an atropine-resistant contraction of isolated airways, which was completely prevented by capsaicin desensitization. Furthermore, NaHS-induced contraction was reduced by TRPV1 antagonism (ruthenium red, capsazepine and SB366791), and was abolished by pretreatment with the combination of tachykinin NK(1) (SR140333) and NK(2) (SR48968) receptor antagonists. In anesthetized guinea-pigs, intratracheal instillation of NaHS increased the total lung resistance and airway plasma protein extravasation. These two effects were reduced by TRPV1 antagonism (capsazepine) and tachykinin receptors (SR140333 and SR48968) blockade. Our results provide the first pharmacological evidence that H(2)S provokes tachykinin-mediated neurogenic inflammatory responses in guinea-pig airways, and that this effect is mediated by stimulation of TRPV1 receptors on sensory nerves endings. This novel mechanism may contribute to the irritative action of H(2)S in the respiratory system.
Figures
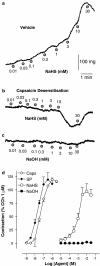
Figure 1
The NaHS-induced release of SP and CGRP LI was significantly reduced following capsaicin desensitization (Caps Des, 10 μ
M
for 60 min) or in the presence of capsazepine (CPZ, 10 μ
M
). Each entry is the mean±s.e.m. of at least five experiments. *P<0.05 vs veh, Dunnett's _t_-test.
Figure 2
Typical traces taken from guinea-pig isolated bronchus representing cumulative concentration-response curves (CRC) to (b) NaHS following capsaicin desensitization (10 μ
M
twice for 20 min, 30 min before the stimulus) or its vehicle (a) and CRC to NaOH (c); (d) pooled data of CRC to capsaicin (Caps), SP, NaHS and NaOH. Each point represents the mean±s.e.m. value of at least six experiments.
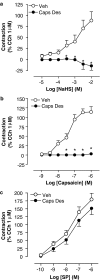
Figure 3
Cumulative CRC to (a) NaHS, (b) capsaicin (Caps) and (c) SP following capsaicin desensitization (Caps Des, 10 μ
M
twice for 20 min, 30 min before the stimulus) or vehicle (veh) pretreatment. Each entry represents the mean±s.e.m. value of at least six experiments (*P<0.05 vs veh).
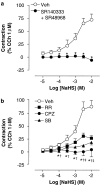
Figure 4
Cumulative CRC to NaHS (a) in the presence of the combination of the tachykinin NK1 (SR140333 1 μ
M
) and NK2 (SR48968, 1 μ
M
) receptor antagonists, (b) the TRPV1 receptor antagonists, ruthenium red (RR, 10 μ
M
), capsazepine (CPZ, 10 μ
M
), SB366791 (1 μ
M
) or the combination of their respective vehicles (veh). Each entry represents the mean±s.e.m. value of at least six experiments (*P<0.05 tachykinin antagonists or CPZ vs veh; †P<0.05 SB366791 vs veh; ‡P<0.05 RR vs veh).
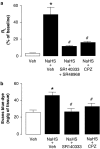
Figure 5
(a) Total _R_L and (b) plasma protein extravasation in guinea-pig trachea after the intratracheal instillation of NaHS (50 m
M
, 200 _μ_l; black column) or its vehicle (white columns). The effects of NaHS were analyzed in the presence or absence of the tachykinin NK1 (SR140333) and/or NK2 (SR48968) receptor antagonist (both at 1.6 _μ_mol kg−1, i.v.), and the TRPV1 receptor antagonists, capsazepine (CPZ, 10 μ
M
). Each entry is the mean±s.e.m. of at least six experiments (*P<0.05 vs veh of NaHS; #P<0.05 vs veh).
Similar articles
- Immunohistochemical co-localization of transient receptor potential vanilloid (TRPV)1 and sensory neuropeptides in the guinea-pig respiratory system.
Watanabe N, Horie S, Michael GJ, Keir S, Spina D, Page CP, Priestley JV. Watanabe N, et al. Neuroscience. 2006 Sep 1;141(3):1533-43. doi: 10.1016/j.neuroscience.2006.04.073. Epub 2006 Jun 12. Neuroscience. 2006. PMID: 16765524 - Acid-induced modulation of airway basal tone and contractility: role of acid-sensing ion channels (ASICs) and TRPV1 receptor.
Faisy C, Planquette B, Naline E, Risse PA, Frossard N, Fagon JY, Advenier C, Devillier P. Faisy C, et al. Life Sci. 2007 Sep 8;81(13):1094-102. doi: 10.1016/j.lfs.2007.08.026. Epub 2007 Sep 4. Life Sci. 2007. PMID: 17869310 - [Effects of tachykinin receptor antagonists on allergic asthma in guinea pigs].
Zhang WP, Lu ZY, Wei EQ. Zhang WP, et al. Yao Xue Xue Bao. 1997 May;32(5):326-30. Yao Xue Xue Bao. 1997. PMID: 11498865 Chinese. - Mechanisms of citric acid-induced bronchoconstriction.
Ricciardolo FL. Ricciardolo FL. Am J Med. 2001 Dec 3;111 Suppl 8A:18S-24S. doi: 10.1016/s0002-9343(01)00816-6. Am J Med. 2001. PMID: 11749919 Review. - The transient receptor potential vanilloid 1: role in airway inflammation and disease.
Geppetti P, Materazzi S, Nicoletti P. Geppetti P, et al. Eur J Pharmacol. 2006 Mar 8;533(1-3):207-14. doi: 10.1016/j.ejphar.2005.12.063. Epub 2006 Feb 7. Eur J Pharmacol. 2006. PMID: 16464449 Review.
Cited by
- Hydrogen sulfide promotes calcium uptake in larval zebrafish.
Kwong RW, Perry SF. Kwong RW, et al. Am J Physiol Cell Physiol. 2015 Jul 1;309(1):C60-9. doi: 10.1152/ajpcell.00053.2015. Epub 2015 May 6. Am J Physiol Cell Physiol. 2015. PMID: 25948733 Free PMC article. - The differential contractile responses to capsaicin and anandamide in muscle strips isolated from the rat urinary bladder.
Saitoh C, Kitada C, Uchida W, Chancellor MB, de Groat WC, Yoshimura N. Saitoh C, et al. Eur J Pharmacol. 2007 Sep 10;570(1-3):182-7. doi: 10.1016/j.ejphar.2007.05.016. Epub 2007 Jun 5. Eur J Pharmacol. 2007. PMID: 17586490 Free PMC article. - H2S biogenesis by human cystathionine gamma-lyase leads to the novel sulfur metabolites lanthionine and homolanthionine and is responsive to the grade of hyperhomocysteinemia.
Chiku T, Padovani D, Zhu W, Singh S, Vitvitsky V, Banerjee R. Chiku T, et al. J Biol Chem. 2009 Apr 24;284(17):11601-12. doi: 10.1074/jbc.M808026200. Epub 2009 Mar 4. J Biol Chem. 2009. PMID: 19261609 Free PMC article. - Transient receptor potential (TRP) channels: a clinical perspective.
Kaneko Y, Szallasi A. Kaneko Y, et al. Br J Pharmacol. 2014 May;171(10):2474-507. doi: 10.1111/bph.12414. Br J Pharmacol. 2014. PMID: 24102319 Free PMC article. Review. - Hydrogen sulfide augments synaptic neurotransmission in the nucleus of the solitary tract.
Austgen JR, Hermann GE, Dantzler HA, Rogers RC, Kline DD. Austgen JR, et al. J Neurophysiol. 2011 Oct;106(4):1822-32. doi: 10.1152/jn.00463.2011. Epub 2011 Jul 6. J Neurophysiol. 2011. PMID: 21734104 Free PMC article.
References
- AMADESI S., NIE J., VERGNOLLE N., COTTRELL G.S., GRADY E.F., TREVISANI M., MANNI C., GEPPETTI P., MC ROBERTS J.A., ENNES H., DAVIS J.B., MAYER E.A., BUNNETT N.W. Protease-activated receptor 2 sensitizes the capsaicin receptor transient receptor potential vanilloid receptor 1 to induce hyperalgesia. J. Neurosci. 2004;24:4300–4312. - PMC - PubMed
- AMANN R., MAGGI C.A. Ruthenium red as a capsaicin antagonist. Life Sci. 1991;49:849–856. - PubMed
- AMDUR M.O., MEAD J. Mechanics of respiration in unanesthetized guinea pigs. Am. J. Physiol. 1958;192:364–368. - PubMed
- BARNES P.J. Neuroeffector mechanisms: the interface between inflammation and neuronal responses. J. Allergy Clin. Immunol. 1996;98:S73–S81. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources