Inflammation and the reciprocal production of granulocytes and lymphocytes in bone marrow - PubMed (original) (raw)
Inflammation and the reciprocal production of granulocytes and lymphocytes in bone marrow
Yoshihiro Ueda et al. J Exp Med. 2005.
Abstract
The coordinated production of leukocytes in bone marrow is crucial for innate and adaptive immunity. Inflammation alters normal leukocyte production by promoting granulopoiesis over lymphopoiesis, a response that supports the reactive neutrophilia that follows infection. Here we demonstrate that this specialization for granulopoiesis is determined by inflammation-induced reductions of growth and retention factors, most significantly stem cell factor and CXCL12, which act preferentially to inhibit lymphoid development. These hierarchical effects suggest that the normal equilibrium of leukocyte production in bone marrow is determined by lymphopoiesis' higher demand for specific growth factors and/or retention signals. Inflammation regulates this balance by reducing growth factors that have less impact on developing neutrophils than lymphocytes. We demonstrate that granulopoiesis and lymphopoiesis are coupled specifically in the bone marrow by development in a common niche and propose that the leukopoietic equilibrium is specified by limiting amounts of developmental resources.
Figures
Figure 1.
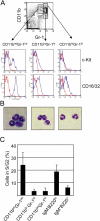
Definition of neutrophil subsets in the BM of naive C57BL/6 mice. BM cells were labeled with mAbs specific for mouse CD11b, CD16/32, c-Kit, or RB6-8C5 Ags. Labeled cells were analyzed/sorted by flow cytometry. Data represent three determinations with two mice/group (n = 6). (A) Expression of CD11b and the RB6-8C5 Ag identifies three populations of double-positive cells: CD11bintGr-1int, CD11bloGr-1hi, and CD11bhiGr-1hi. These populations expressed distinct levels of CD40, c-Kit, F4/80, and CD16/32 (blue) as compared with nonspecific, isotype-matched control Abs (red). (B) Sorted CD11bintGr-1int, CD11bloGr-1hi, and CD11bhiGr-1hi BM cells after Giemsa staining. CD11bintGr-1int cells exhibit the morphology of promyelocytes and myelocytes; CD11bloGr-1hi and CD11bhiGr-1hi cells represent increasingly mature cell forms. (C) Fraction of BM neutrophil and B cell populations in the G2 and S phases of the cell cycle. Among the BM granulocyte compartments, only CD11bintGr-1int promyelocytes and myelocytes exhibit significant levels of mitosis. The DNA content of single cells in each phenotypic compartment was determined by intracellular propidium iodide staining. Bars represent the average fraction (±SD) of cells in the G2 and S phases of the cell cycle.
Figure 2.
Immunization with adjuvant depletes mature, but not immature, neutrophils from BM. (A) Population dynamics of BM neutrophil and B cell subsets after immunization. 4 to 16 d after immunization, BM cells from C57BL/6 mice (n = 4–6) were recovered and labeled with mAb specific for CD11b, the RB6-8C5 Ag, or B220. CD11bintGr-1int, CD11bloGr-1hi, and CD11bhiGr-1hi neutrophils and B220lo and B220hi lymphocytes were enumerated by flow cytometry to determine the effects of immunization on each population. CD11bintGr-1int and CD11bloGr-1hi cell numbers expand and contract reciprocally to B220lo B cells. Points represent average (±SEM) numbers of Gr-1int (•), Gr-1hi (▪), B220lo (○), or B220hi (□) cells from a tibia and femur at the times indicated; naive controls are shown (day 0). (B) FACS profiles of CD11b+Gr-1+ and B220+ BM cell populations before (left), and 4 d after (right) immunization represent three independent experiments with two mice/group (n = 6). Immunization reduces the CD11bhiGr-1hi neutrophil subset and both B220lo and B220hi lymphocytes, but expands CD11bintGr-1int and CD11bloGr-1hi cell sets.
Figure 3.
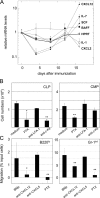
Inflammation modulates the expression of lymphoid, but not myeloid, growth factors in the BM. (A) Immunization strongly (>10-fold) reduced steady-state levels of mRNA specific for CXCL12 and SCF; relative to HPRT, BAFF and IL-7 message levels were not suppressed significantly. By 16 d after immunization, mRNA levels for lymphocyte growth factors were supranormal. mRNA specific for various growth factors was quantitated in reference to GAPDH message present in the same sample. Each point (n = 2–5) represents the average (±SD) amount of specific mRNA relative to mean levels present in naive controls. Levels of mRNA for GM-CSF and IL-3 were below the limits of detection. (B) Reduced c-Kit signaling preferentially inhibits proliferation/survival and differentiation of CLP over CMP. 100 CLP or CMP were cultured on adherent BM cell layers in media supplemented with hematopoietic growth factors (n = 2–3). PTX, or mAb specific for LFA-1 or c-Kit were added to cultures to determine the effect of reduced c-Kit signaling on lymphopoiesis and myelopoiesis. PTX inhibits lymphopoiesis and myelopoiesis equally but antagonistic anti–c-Kit mAb preferentially suppressed only the CLP cultures. (C) Preferential inhibition of chemotaxis of B220lo and Gr-1int BM cells by anti-CXCL12 mAb. In vitro, B220lo and Gr-1int BM cells migrate to BMp. This migration is abrogated completely by PTX and unaffected by mAb to CXCL5 (n = 2). However, mAb specific for CXCL12 suppresses the migration of B220lo cells (13% of BMp control) more than Gr-1int cells (50% of BMp control; n = 5–7). *P ≤ 0.05; **P≤ 0.01.
Figure 4.
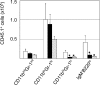
The ability of immature, CD11bintGr-1int and CD11bloGr-1hi granulocytes to home to the BM is resistant to PTX. BM cells from B6.SJL (CD45.1) mice were exposed to PTX, 0 ng/ml (n = 3; open bars); 25 ng/ml (n = 4; filled bars); and 100 ng/ml (n = 2; stippled bars) and injected into C57BL/6 (CD45.2) recipients. 14 h later, CD45.1+ cells in host BM were enumerated by flow cytometry. Bars represent mean (±SD) numbers of CD45.1+ cells; significant (P < 0.05) changes from controls are indicated (*). Whereas exposure to 25 ng/ml PTX significantly reduced the numbers of transferred CD11bhiGr-1hi neutrophils and B220lo lymphocytes, homing by CD11bintGr-1int and CD11bloGr-1hi granulocytes was inhibited insignificantly (P > 0.05), even after exposure to 100 ng/ml PTX.
Figure 5.
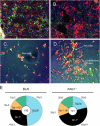
BM granulocytes and B lymphocytes share a common developmental niche. (A) The femoral BM of naive mice contains many closely associated Gr-1+ (red) and B220+ (green) cell clusters. (B) 4 d after immunization with adjuvants, the size of Gr-1+ BM cell clusters is much increased; this expansion includes areas that formerly were occupied by B220+ lymphocytes (see Fig. 1). (C) Culture of c-Kit+Gr-1+ (red) and c-Kit+B220+ P (green) in the absence of BM stromal cell layers results in little or no proliferation and very limited development. (D) In the presence of stromal cell layers, c-Kit+Gr-1+ and c-Kit+B220+ cells migrate beneath stromal cell bodies to form cobblestone clusters that contain lymphocyte- and granulocyte precursors. (E) Hematopoietic compartments in the BM of Rag-1 −/− and congenic C57BL/6 mice. Femoral BM cells from C57BL/6 (n = 5) and RAG1-deficient mice (n = 5) were labeled with the Gr-1 mAb and mAbs specific for B220 and TER119; labeled and unlabeled BM cells from individual femurs were enumerated by flow cytometry. Control femurs contained an average of 156 × 105 nucleated cells (center), comprising ∼76 × 105 B220+ cells, 43 × 105 Gr-1+ cells, 18 × 105 TER119+ cells, and 19 × 105 unlabeled cells (outer segments). Femoral BM cell numbers in Rag-1 −/− mice (center; 31 × 105) were significantly (P = 0.002) lower than controls with losses confined to B220+ compartments. Femurs of RAG1-deficient mice contained an average of only 28 × 105 B220+ cells but a compensating increase of Gr-1+ cell numbers to 63 × 105 cells/femur. In contrast, RAG1-deficient and control mice contained virtually identical numbers of TER119+ and unlabeled cells.
Figure 6.
Models for interaction between the B220+, Gr-1+, and TER119+ hematopoietic compartments of BM. In normal femoral BM, B220+ lymphocytes, Gr-1+ granulocytes, and TER119+ erythroid cells are present in stable ratios (4:2:1; Fig. 5 E) that define one of three possible relationships between these hematopoietic compartments: independence, general dependence, or specific dependence. In the case of independence (left), growth resources for each compartment are distinct and the compartments do not compete. Under these conditions, removal of the large B220+ compartment by a lymphocyte-specific, developmental blockade would not alter the size/state of the other cell compartments. If all hematopoietic cell compartments share growth resources and are generally dependent (center), reductions in B220+ cell numbers would result in compensatory increases in the Gr-1 and TER119 compartments. Specific dependence (right) between hematopoietic compartments exists when some, but not all, compartments compete for a growth resources. If the B220+ and Gr-1+ BM compartments share a developmental niche that excludes the erythroid lineages, depletion of the B220+ cells would result in compensatory increases in Gr-1 cell numbers without changes in the TER119 compartment. This outcome is observed in the BM of RAG1-deficient mice (Fig. 5 E).
Similar articles
- Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1.
Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Nagasawa T, et al. Nature. 1996 Aug 15;382(6592):635-8. doi: 10.1038/382635a0. Nature. 1996. PMID: 8757135 - TL1A and IL-18 synergy promotes GM-CSF-dependent thymic granulopoiesis in mice.
Ruiz Pérez M, Maueröder C, Steels W, Verstraeten B, Lameire S, Xie W, Wyckaert L, Huysentruyt J, Divert T, Roelandt R, Gonçalves A, De Rycke R, Ravichandran K, Lambrecht BN, Taghon T, Leclercq G, Vandenabeele P, Tougaard P. Ruiz Pérez M, et al. Cell Mol Immunol. 2024 Aug;21(8):807-825. doi: 10.1038/s41423-024-01180-8. Epub 2024 Jun 5. Cell Mol Immunol. 2024. PMID: 38839915 Free PMC article. - Impairment of lymphopoiesis and myelopoiesis in mice reconstituted with bone marrow-hematopoietic progenitor cells expressing SDF-1-intrakine.
Onai N, Zhang Yy, Yoneyama H, Kitamura T, Ishikawa S, Matsushima K. Onai N, et al. Blood. 2000 Sep 15;96(6):2074-80. Blood. 2000. PMID: 10979950 - Chemokines in lymphopoiesis and lymphoid organ development.
Ansel KM, Cyster JG. Ansel KM, et al. Curr Opin Immunol. 2001 Apr;13(2):172-9. doi: 10.1016/s0952-7915(00)00201-6. Curr Opin Immunol. 2001. PMID: 11228410 Review. - Neutrophil Homeostasis and Emergency Granulopoiesis: The Example of Systemic Juvenile Idiopathic Arthritis.
Malengier-Devlies B, Metzemaekers M, Wouters C, Proost P, Matthys P. Malengier-Devlies B, et al. Front Immunol. 2021 Dec 13;12:766620. doi: 10.3389/fimmu.2021.766620. eCollection 2021. Front Immunol. 2021. PMID: 34966386 Free PMC article. Review.
Cited by
- Mice Deficient in NOX2 Display Severe Thymic Atrophy, Lymphopenia, and Reduced Lymphopoiesis in a Zymosan-Induced Model of Systemic Inflammation.
Sugimoto Y, Endo D, Aratani Y. Sugimoto Y, et al. Inflammation. 2021 Feb;44(1):371-382. doi: 10.1007/s10753-020-01342-6. Epub 2020 Sep 16. Inflammation. 2021. PMID: 32939668 - Enhancement of hematopoiesis and lymphopoiesis in diet-induced obese mice.
Trottier MD, Naaz A, Li Y, Fraker PJ. Trottier MD, et al. Proc Natl Acad Sci U S A. 2012 May 15;109(20):7622-9. doi: 10.1073/pnas.1205129109. Epub 2012 Apr 25. Proc Natl Acad Sci U S A. 2012. PMID: 22538809 Free PMC article. - Plasticity of Myeloid Cells during Oral Barrier Wound Healing and the Development of Bisphosphonate-related Osteonecrosis of the Jaw.
Sun Y, Kaur K, Kanayama K, Morinaga K, Park S, Hokugo A, Kozlowska A, McBride WH, Li J, Jewett A, Nishimura I. Sun Y, et al. J Biol Chem. 2016 Sep 23;291(39):20602-16. doi: 10.1074/jbc.M116.735795. Epub 2016 Aug 11. J Biol Chem. 2016. PMID: 27514746 Free PMC article. - Pertussis toxin-sensitive G proteins regulate lymphoid lineage specification in multipotent hematopoietic progenitors.
Lai AY, Watanabe A, O'Brien T, Kondo M. Lai AY, et al. Blood. 2009 Jun 4;113(23):5757-64. doi: 10.1182/blood-2009-01-201939. Epub 2009 Apr 10. Blood. 2009. PMID: 19363218 Free PMC article. - Infection-induced changes in hematopoiesis.
Glatman Zaretsky A, Engiles JB, Hunter CA. Glatman Zaretsky A, et al. J Immunol. 2014 Jan 1;192(1):27-33. doi: 10.4049/jimmunol.1302061. J Immunol. 2014. PMID: 24363432 Free PMC article. Review.
References
- Glasser, L., and R.L. Fiederlein. 1987. Functional differentiation of normal human neutrophils. Blood. 69:937–944. - PubMed
- Quie, P.G. 1980. The phagocytic system in host defense. Scand J Infect Dis Suppl. 24:30–32. - PubMed
- Burg, N.D., and M.H. Pillinger. 2001. The neutrophil: function and regulation in innate and humoral immunity. Clin. Immunol. 99:7–17. - PubMed
- Engle, W.D., and C.R. Rosenfeld. 1984. Neutropenia in high-risk neonates. J. Pediatr. 105:982–986. - PubMed
- Gessler, P., R. Luders, S. Konig, N. Haas, P. Lasch, and W. Kachel. 1995. Neonatal neutropenia in low birthweight premature infants. Am. J. Perinatol. 12:34–38. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI056123/AI/NIAID NIH HHS/United States
- R01 AI024335/AI/NIAID NIH HHS/United States
- CA-98129/CA/NCI NIH HHS/United States
- AI-56123/AI/NIAID NIH HHS/United States
- AI-52077/AI/NIAID NIH HHS/United States
- U19 AI056363/AI/NIAID NIH HHS/United States
- T32 AI052077/AI/NIAID NIH HHS/United States
- AI-56363/AI/NIAID NIH HHS/United States
- R01 CA098129/CA/NCI NIH HHS/United States
- AI-49326/AI/NIAID NIH HHS/United States
- AI-24335/AI/NIAID NIH HHS/United States
- R01 AI049326/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources