Phosphorylation of human oxoguanine DNA glycosylase (alpha-OGG1) modulates its function - PubMed (original) (raw)
Phosphorylation of human oxoguanine DNA glycosylase (alpha-OGG1) modulates its function
Jingping Hu et al. Nucleic Acids Res. 2005.
Abstract
Oxoguanine DNA glycosylase (OGG1) initiates the repair of 8-oxoguanine (8-oxoG), a major oxidative DNA base modification that has been directly implicated in cancer and aging. OGG1 functions in the base excision repair pathway, for which a molecular hand-off mechanism has been proposed. To date, only one functional and a few physical protein interactions have been reported for OGG1. Using the yeast two-hybrid system and a protein array membrane, we identified two novel protein interactions of OGG1, with two different protein kinases: Cdk4, a serine-threonine kinase, and c-Abl, a tyrosine kinase. We confirmed these interactions in vitro using recombinant proteins and in vivo by co-immunoprecipitation from whole cell extracts. OGG1 is phosphorylated in vitro by Cdk4, resulting in a 2.5-fold increase in the 8-oxoG/C incision activity of OGG1. C-Abl tyrosine phosphorylates OGG1 in vitro; however, this phosphorylation event does not affect OGG1 8-oxoG/C incision activity. These results provide the first evidence that a post-translational modification of OGG1 can affect its catalytic activity. The distinct functional outcomes from serine/threonine or tyrosine phosphorylation may indicate that activation of different signal transduction pathways modulate OGG1 activity in different ways.
Figures
Figure 1
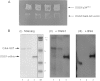
Physical interaction between OGG1 and Cdk4. (A) Yeast strain AH109 [Matchmaker™ Gal4 Two-hybrid system 3 (Clontech)] was transformed with pGBKT7-_OGG1_-α and p34SEI-1 or empty Gal4-AD vector. The yeast were cultured in high-stringency selective meduim and allowed to grow for 1 week. (B) Recombinant his-tagged OGG1 (OGG1-6his) and GST-tagged Cdk4 (Santa Cruz Biotech.) were separated on 12% Tris–glycine polyacrylamide gels. One gel was stained with Coomassie blue and the others were electro-transferred to PVDF membranes. The membranes were then incubated with either purified OGG1-6his (+OGG1) or BSA (+BSA) and probed with anti-OGG1 antibody, as described in Materials and Methods. Lanes 1 and 2 contain 800 and 400 ng Cdk4-GST protein; lane 3 contains 450 ng OGG1-6his in the Coomassie gel and 10 ng in the immunoblots.
Figure 2
OGG1 and Cdk4 interact in vivo. HEK293 cells were transfected with FLAG-tagged OGG1 plasmid or empty vector. After 2 weeks selection with G418 (Invitrogen), whole cell extracts were prepared from the pool of cells as described in Materials and Methods. A monoclonal antibody, anti-FLAG-M2 agarose (Sigma), was used to immunoprecipitate FLAG-tagged OGG1. The immunoprecipitates were separated on 12% Tris-glycine PAGE gels and analyzed by western blot with the indicated antibodies.
Figure 3
In vitro phosphorylation of OGG1 by Cdk4. (A) Recombinant OGG1-6his (62, 125, 250, 500 and 1000 ng), purified from insect cell (Sf9), was immunobloted with an anti-phosphoserine antibody. (B) An aliquot of 100 ng of purified OGG1 was incubated with either active immunoprecipitated Cdk4 beads (lanes 1 and 3) or heat-inactivated immunoprecipitated Cdk4 beads (lane 2) in the presence of [γ-32P]ATP. One sample (lane 1) was then subjected to PP1 treatment after Cdk4 phosphorylation. The phosphorylated proteins were analyzed by autoradiography, followed by electro-blotting of the same gel to PVDF membrane, and western blot analysis with the indicated antibodies.
Figure 4
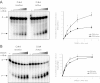
OGG1 phosphorylation by Cdk4 increases 8-oxoG strand cleavage and base release activities. 10 ng of purified OGG1-α-6his was first incubated with PP1 to ensure complete dephosphorylation. The proteins were then incubated with immunoprecipitated Cdk4, or heat-inactivated Cdk4 as a negative control. (A) Serial dilutions of OGG1-α-6his (0.25, 0.5, 1, 2 and 4 ng) were analyzed for 8-oxoG strand cleavage activity measurement, as described in Materials and Methods. (B) Similar OGG1-α-6his concentrations were analyzed for base release activity. The graphs on the right side of each panel present the average ±SEM of at least two independent replicates. Asterisks, significantly higher than inactive Cdk4 at P < 0.05.
Figure 5
PKC phosphorylates OGG1 in vitro but does not change its incision activity. (A) An aliquot of 50–100 ng of recombinant OGG1-6his was incubated with or without 5 ng PKC (Promega) in the presence of [γ-32P]ATP. The reactions were separated by SDS–PAGE and analyzed by autoradiography (top panel) and western blotting (bottom panel). (B) An aliquot of 0.25–6 ng of purified OGG1-α-6his was incubated with or without 5 ng PKC and 8-oxoG incision activity was measured as described in Materials and Methods. The graph presents the average ±SEM of two independent replicates.
Figure 6
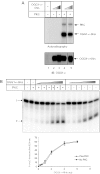
c-Abl physically interacts with and phosphorylates OGG1 in vitro. (A) An aliquot of 25, 50 and 100 ng of Cdk4, XRCC1, c-Abl, WRN and hOGG1 were applied onto the DiscoverLight™ Protein-array membrane. The membrane was processed and developed as described in the Materials and Methods. As positive controls, we blotted 1 μl of 293-FLAG-OGG1 lysate and a horseradish peroxidase control provided by the manufacturer. (B) Purified OGG1-α-6his was incubated with either GST (lane 1) or GST-c-Abl fusion proteins (inactivated in lane 2, active in lane 3). GST-pull down was performed as described in Materials and Methods. The proteins were then detected by immunoblot. OGG1-α-6his was loaded in lane 4 as a positive control. (C) Purified OGG1-6his was incubated with 100 ng full-length c-Abl either in the presence (lanes 2–4) or absence of ATP (lanes 5–7) to look at tyrosine phosphorylation. The proteins were analyzed by immunoblot with anti-phosphotyrosine (middle panel) followed by anti-OGG1 (top panel) and anti-c-Abl (bottom panel) antibodies.
Figure 7
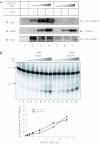
c-Abl phosphorylation of OGG1 does not change 8-oxoG incision. (A) Purified OGG1-6his protein (12.5, 25 and 50 ng) was incubated with full-length c-Abl (100 ng in each reaction), either active (lanes 2–4, allowing tyrosine phosphorylation) or heat-inactivated (lanes 5–7, unphosphorylated). The proteins were then analyzed by western blotting with the specified antibodies. (B) Purified OGG1-6his (0.25–12 ng) was incubated with 100 ng active (lanes 3–8) or heat-inactivated (lanes 9–14) c-Abl and then used for an 8-oxoG incision activity assay, as described in Materials and Methods. The graph presented shows the average ±SEM of at least two independent replicates.
Similar articles
- The C-terminal alphaO helix of human Ogg1 is essential for 8-oxoguanine DNA glycosylase activity: the mitochondrial beta-Ogg1 lacks this domain and does not have glycosylase activity.
Hashiguchi K, Stuart JA, de Souza-Pinto NC, Bohr VA. Hashiguchi K, et al. Nucleic Acids Res. 2004 Oct 19;32(18):5596-608. doi: 10.1093/nar/gkh863. Print 2004. Nucleic Acids Res. 2004. PMID: 15494448 Free PMC article. - Stimulation of DNA glycosylase activity of OGG1 by NEIL1: functional collaboration between two human DNA glycosylases.
Mokkapati SK, Wiederhold L, Hazra TK, Mitra S. Mokkapati SK, et al. Biochemistry. 2004 Sep 14;43(36):11596-604. doi: 10.1021/bi049097i. Biochemistry. 2004. PMID: 15350146 - Silver nanoparticles down-regulate Nrf2-mediated 8-oxoguanine DNA glycosylase 1 through inactivation of extracellular regulated kinase and protein kinase B in human Chang liver cells.
Piao MJ, Kim KC, Choi JY, Choi J, Hyun JW. Piao MJ, et al. Toxicol Lett. 2011 Nov 30;207(2):143-8. doi: 10.1016/j.toxlet.2011.09.002. Epub 2011 Sep 8. Toxicol Lett. 2011. PMID: 21925250 - An active alternative splicing isoform of human mitochondrial 8-oxoguanine DNA glycosylase (OGG1).
Furihata C. Furihata C. Genes Environ. 2015 Oct 1;37:21. doi: 10.1186/s41021-015-0021-9. eCollection 2015. Genes Environ. 2015. PMID: 27350816 Free PMC article. Review. - Roles of DNA repair enzyme OGG1 in innate immunity and its significance for lung cancer.
Vlahopoulos S, Adamaki M, Khoury N, Zoumpourlis V, Boldogh I. Vlahopoulos S, et al. Pharmacol Ther. 2019 Feb;194:59-72. doi: 10.1016/j.pharmthera.2018.09.004. Epub 2018 Sep 19. Pharmacol Ther. 2019. PMID: 30240635 Free PMC article. Review.
Cited by
- Oxygen consumption and usage during physical exercise: the balance between oxidative stress and ROS-dependent adaptive signaling.
Radak Z, Zhao Z, Koltai E, Ohno H, Atalay M. Radak Z, et al. Antioxid Redox Signal. 2013 Apr 1;18(10):1208-46. doi: 10.1089/ars.2011.4498. Epub 2012 Nov 16. Antioxid Redox Signal. 2013. PMID: 22978553 Free PMC article. Review. - Yeast apurinic/apyrimidinic endonuclease Apn1 protects mammalian neuronal cell line from oxidative stress.
Ho R, Rachek LI, Xu Y, Kelley MR, LeDoux SP, Wilson GL. Ho R, et al. J Neurochem. 2007 Jul;102(1):13-24. doi: 10.1111/j.1471-4159.2007.04490.x. Epub 2007 May 15. J Neurochem. 2007. PMID: 17506861 Free PMC article. - The role of base excision repair genes OGG1, APN1 and APN2 in benzo[a]pyrene-7,8-dione induced p53 mutagenesis.
Abedin Z, Louis-Juste M, Stangl M, Field J. Abedin Z, et al. Mutat Res. 2013 Jan 20;750(1-2):121-8. doi: 10.1016/j.mrgentox.2012.10.003. Epub 2012 Oct 29. Mutat Res. 2013. PMID: 23117049 Free PMC article. - 8-Oxoguanine DNA glycosylase 1: Beyond repair of the oxidatively modified base lesions.
Ba X, Boldogh I. Ba X, et al. Redox Biol. 2018 Apr;14:669-678. doi: 10.1016/j.redox.2017.11.008. Epub 2017 Nov 10. Redox Biol. 2018. PMID: 29175754 Free PMC article. Review. - Intranuclear matrix metalloproteinases promote DNA damage and apoptosis induced by oxygen-glucose deprivation in neurons.
Hill JW, Poddar R, Thompson JF, Rosenberg GA, Yang Y. Hill JW, et al. Neuroscience. 2012 Sep 18;220:277-90. doi: 10.1016/j.neuroscience.2012.06.019. Epub 2012 Jun 16. Neuroscience. 2012. PMID: 22710064 Free PMC article.
References
- Dizdaroglu M., Jaruga P., Birincioglu M., Rodriguez H. Free radical-induced damage to DNA: mechanisms and measurement. Free Radic. Biol. Med. 2002;32:1102–1115. - PubMed
- Lindahl T. Instability and decay of the primary structure of DNA. Nature. 1993;362:709–715. - PubMed
- Grollman A.P., Moriya M. Mutagenesis by 8-oxoguanine: an enemy within. Trends Genet. 1993;9:246–249. - PubMed
- Barja G. Aging in vertebrates, and the effect of caloric restriction: a mitochondrial free radical production-DNA damage mechanism? Biol. Rev. Camb. Philos. Soc. 2004;79:235–251. - PubMed
- Dianov G.L., Souza-Pinto N., Nyaga S.G., Thybo T., Stevnsner T., Bohr V.A. Base excision repair in nuclear and mitochondrial DNA. Prog. Nucleic Acid Res. Mol. Biol. 2001;68:285–297. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous