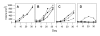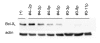The anti-tumor effect of Apo2L/TRAIL on patient pancreatic adenocarcinomas grown as xenografts in SCID mice - PubMed (original) (raw)
The anti-tumor effect of Apo2L/TRAIL on patient pancreatic adenocarcinomas grown as xenografts in SCID mice
Bonnie L Hylander et al. J Transl Med. 2005.
Abstract
BACKGROUND: Apo2L/TRAIL has considerable promise for cancer therapy based on the fact that this member of the tumor necrosis factor family induces apoptosis in the majority of malignant cells, while normal cells are more resistant. Furthermore, in many cells, when Apo2L/TRAIL is combined with chemotherapy, the effect is synergistic. The majority of this work has been carried out using cell lines. Therefore, investigation of how patient tumors respond to Apo2L/TRAIL can validate and/or complement information obtained from cell lines and prove valuable in the design of future clinical trials. METHODS: We have investigated the Apo2L/TRAIL sensitivity of patient derived pancreatic tumors using a patient tumor xenograft/ SCID mouse model. Mice bearing engrafted tumors were treated with Apo2L/TRAIL, gemcitabine or a combination of both therapies. RESULTS: Patient tumors grown as xenografts exhibited a spectrum of sensitivity to Apo2L/TRAIL. Both Apo2L/TRAIL sensitive and resistant pancreatic tumors were found, as well as tumors that showed heterogeneity of response. Changes in apoptotic signaling molecules in a sensitive tumor were analyzed by Western blot following Apo2L/TRAIL treatment; loss of procaspase 8, Bid and procaspase 3 was observed and correlated with inhibition of tumor growth. However, in a tumor that was highly resistant to killing by Apo2L/TRAIL, although there was a partial loss of procaspase 8 and Bid in response to Apo2L/TRAIL treatment, loss of procaspase 3 was negligible. This resistant tumor also expressed a high level of the anti-apoptotic molecule Bcl-XL that, in comparison, was not detected in a sensitive tumor. Importantly, in the majority of these tumors, addition of gemcitabine to Apo2L/TRAIL resulted in a greater anti-tumor effect than either therapy used alone. CONCLUSION: These data suggest that in a clinical setting we will see heterogeneity in the response of patients' tumors to Apo2L/TRAIL, including tumors that are highly sensitive as well as those that are resistant. While much more work is needed to understand the molecular basis for this heterogeneity, it is very encouraging, that Apo2L/TRAIL in combination with gemcitabine increased therapeutic efficacy in almost every case and therefore may be a highly effective strategy for controlling human pancreatic cancer validating and expanding upon what has been reported for cell lines.
Figures
Figure 1
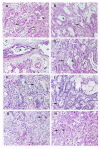
The histological features of patient pancreatic adenocarcinomas (left hand panels: A, C, E, G) are maintained when these tumors are grown as xenografts (right hand panels: B, D, F, H) in SCID mice. The well differentiated glands (arrows) containing secretory material (double arrows) which are seen in surgical specimens, proliferate in xenografts of the same tumors. (Tumor #1: A, B; Tumor #2: C, D; Tumor #3: E, F; Tumor #4: G, H). Original magnification, ×10.
Figure 2
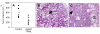
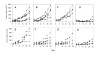
Apo2L/TRAIL significantly inhibited the growth of pancreatic adenocarcinoma #1. Mice bearing patient tumor xenografts were treated with Apo2L/TRAIL and the tumor volumes on the final day of treatment (day 35) were compared (7 mice/group, the mean is indicated by the horizontal line; p = 0.004). B, C: Comparison of the histology of the median tumors. B. In the periphery of an untreated tumor, there are numerous large glands (arrow) containing secretory product. C. The treated tumor has fewer glands (arrow) and a higher proportion of connective tissue. There are also large pools of residual secretory product (S) that are not surrounded by epithelial cells.
Figure 3
Growth of Tumor #2 is also significantly inhibited during treatment with Apo2L/TRAIL. A. Control; B. Apo2L/TRAIL treated. (4 mice/group; day 35, p = 0.016). Following cessation of treatment (arrows), growth of treated tumors progressed at a rate similar to that of the untreated tumors.
Figure 4
Evaluation of the response of Tumor #2 to increasing doses of gemcitabine. A. Control; B. Tumors in mice treated with 1.0 mg/kg gemcitabine grow at a rate comparable to that of untreated tumors; C. Tumors in mice treated with 1.5 mg/kg gemicitabine show growth inhibition to varying degrees (p = .045); D. Tumors in mice treated with 2.5 mg/kg gemcitabine alone show uniform suppression (p < .001).
Figure 5
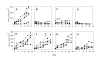
The effect of Apo2L/TRAIL in combination with gemcitabine on an Apo2L/TRAIL sensitive and resistant pancreatic adenocarcinoma. A-D. Tumor #3; E-H. Tumor #4. Groups of tumor bearing mice were treated with: A&E- Controls, B&F- Apo2L/TRAIL alone, C&G- gemcitabine alone, D&H- Apo2L/TRAIL and gemcitabine in combination. Tumor #3 is sensitive to Apo2L/TRAIL alone (B; p = 0.006) while Tumor #4 is resistant to Apo2L/TRAIL (F). This differential sensitivity is also seen in the response to gemcitabine alone (C, p = 0.02, and G). The efficacy of Apo2L/TRAIL in combination with gemcitabine appears to be enhanced in Tumor #3 (D) and is significantly greater than either treatment alone in Tumor #4 (H; p = 0.008).
Figure 6
The anti-tumor effect of Apo2L/TRAIL and gemcitabine in combination is greater than that of either single agent alone. Mice were engrafted with two different patient tumors: A-D, Tumor #2; E-H, Tumor #5. Treatment: A, E. Control; B, F. Apo2L/TRAIL; C, G. gemcitabine; D, H. combination therapy. Although tumors showed variable degrees of sensitivity to either Apo2L/TRAIL or gemcitabine alone, the combination treatment resulted in significant inhibition of tumor growth (D, p = 0.035; H, p = 0.05).
Figure 7
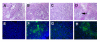
The effect of Apo2L/TRAIL and gemcitabine alone and in combination (Tumor #2, day 5 of treatment). A. Many large glands are apparent in an untreated tumor. B, C. Tumors treated with Apo2L/TRAIL (B) or gemicitabine (C) contain fewer, smaller glands. D. Tumors treated with a combination of Apo2L/TRAIL and gemcitabine consist mainly of fibrotic tissue with foci of residual tumor cells (D, arrow). E-H. TUNEL assay. Few apoptotic cells are apparent in either the untreated (E) or gemcitabine treated (G) treated tumors. The Apo2L/TRAIL (F) and combination treated (H) tumors contain numerous cells undergoing apoptosis.
Figure 8
The effect of Apo2L/TRAIL on levels of procaspase 8, Bid, a marker of intact mitochondria, and procaspase 3 in Tumor #3 (Apo2L/TRAIL sensitive) and Tumor #4 (Apo2L/TRAIL resistant). Two tumors were removed from each group shown in Figure 5 on day 2 of treatment. The lanes are: (+) - positive control for each antibody; C- two tumors from the control group; T- two tumors from the Apo2L/TRAIL treated group; A. The Apo2L/TRAIL sensitive Tumor #3. By the second day of treatment with Apo2L/TRAIL, the levels of procaspase 8, Bid, the mitochondrial marker and procaspase 3 (outlined) in these tumors are greatly reduced. B. The Apo2L/TRAIL resistant Tumor #4. In the tumor that was resistant to Apo2L/TRAIL treatment, although there is a slightly diminished amount of procaspase 8 and Bid present in these tumors following 2 days of treatment with Apo2L/TRAIL, the levels of the mitochondrial marker and procaspase 3 (outlined) remain comparable to the controls. (In A and B a representative actin loading control is shown for each tumor).
Figure 9
Comparison of Bcl-XL expression in a Tumor #4 that is resistant to Apo2L/TRAIL and Tumor #3 that is sensitive. Bcl-XL is detectable by Western blot in several passages of Tumor #4 (2nd passage, 5th passage) while Bcl-XL is not detectable in several passages of Tumor #3 (3rd passage, 8th passage, 11th passage).
Similar articles
- Doxorubicin increases the effectiveness of Apo2L/TRAIL for tumor growth inhibition of prostate cancer xenografts.
El-Zawahry A, McKillop J, Voelkel-Johnson C. El-Zawahry A, et al. BMC Cancer. 2005 Jan 7;5:2. doi: 10.1186/1471-2407-5-2. BMC Cancer. 2005. PMID: 15638938 Free PMC article. - Synergism of CPT-11 and Apo2L/TRAIL against two differentially sensitive human colon tumor xenografts.
Sugamura K, Gibbs JF, Belicha-Villanueva A, Andrews C, Repasky EA, Hylander BL. Sugamura K, et al. Oncology. 2008;74(3-4):188-97. doi: 10.1159/000151366. Epub 2008 Aug 20. Oncology. 2008. PMID: 18714167 Free PMC article. - Expression of anti-apoptotic factors modulates Apo2L/TRAIL resistance in colon carcinoma cells.
Lippa MS, Strockbine LD, Le TT, Branstetter DG, Strathdee CA, Holland PM. Lippa MS, et al. Apoptosis. 2007 Aug;12(8):1465-78. doi: 10.1007/s10495-007-0076-6. Apoptosis. 2007. PMID: 17440816 - The potential of the tumor microenvironment to influence Apo2L/TRAIL induced apoptosis.
Mace TA, Yamane N, Cheng J, Hylander BL, Repasky EA. Mace TA, et al. Immunol Invest. 2006;35(3-4):279-96. doi: 10.1080/08820130600745463. Immunol Invest. 2006. PMID: 16916755 Review. - Targeting the Apo2L/TRAIL system for the therapy of autoimmune diseases and cancer.
Martinez-Lostao L, Marzo I, Anel A, Naval J. Martinez-Lostao L, et al. Biochem Pharmacol. 2012 Jun 1;83(11):1475-83. doi: 10.1016/j.bcp.2011.12.036. Epub 2012 Jan 2. Biochem Pharmacol. 2012. PMID: 22230480 Review.
Cited by
- Establishment of patient-derived non-small cell lung cancer xenograft models with genetic aberrations within EGFR, KRAS and FGFR1: useful tools for preclinical studies of targeted therapies.
Zhang XC, Zhang J, Li M, Huang XS, Yang XN, Zhong WZ, Xie L, Zhang L, Zhou M, Gavine P, Su X, Zheng L, Zhu G, Zhan P, Ji Q, Wu YL. Zhang XC, et al. J Transl Med. 2013 Jul 10;11:168. doi: 10.1186/1479-5876-11-168. J Transl Med. 2013. PMID: 23842453 Free PMC article. - Enhancing the antitumor efficacy of a cell-surface death ligand by covalent membrane display.
Nair PM, Flores H, Gogineni A, Marsters S, Lawrence DA, Kelley RF, Ngu H, Sagolla M, Komuves L, Bourgon R, Settleman J, Ashkenazi A. Nair PM, et al. Proc Natl Acad Sci U S A. 2015 May 5;112(18):5679-84. doi: 10.1073/pnas.1418962112. Epub 2015 Apr 20. Proc Natl Acad Sci U S A. 2015. PMID: 25902490 Free PMC article. - DCE-MRI detects early vascular response in breast tumor xenografts following anti-DR5 therapy.
Kim H, Folks KD, Guo L, Stockard CR, Fineberg NS, Grizzle WE, George JF, Buchsbaum DJ, Morgan DE, Zinn KR. Kim H, et al. Mol Imaging Biol. 2011 Feb;13(1):94-103. doi: 10.1007/s11307-010-0320-2. Mol Imaging Biol. 2011. PMID: 20383593 Free PMC article. - The promise of TRAIL--potential and risks of a novel anticancer therapy.
Koschny R, Walczak H, Ganten TM. Koschny R, et al. J Mol Med (Berl). 2007 Sep;85(9):923-35. doi: 10.1007/s00109-007-0194-1. Epub 2007 Apr 17. J Mol Med (Berl). 2007. PMID: 17437073 Review. - Apoptosis pathways and their therapeutic exploitation in pancreatic cancer.
Fulda S. Fulda S. J Cell Mol Med. 2009 Jul;13(7):1221-7. doi: 10.1111/j.1582-4934.2009.00748.x. Epub 2009 Mar 27. J Cell Mol Med. 2009. PMID: 19382915 Free PMC article. Review.
References
- Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics, 2003. CA Cancer J Clin. 2003;53:5–26. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials