Different cytokine response of primary colonic epithelial cells to commensal bacteria - PubMed (original) (raw)
Different cytokine response of primary colonic epithelial cells to commensal bacteria
Jing-Gang Lan et al. World J Gastroenterol. 2005.
Abstract
Aim: To determine if primary murine colonic epithelial cells (CEC) respond to commensal bacteria and discriminate between different types of bacteria.
Methods: A novel CEC: bacteria co-culture system was used to compare the ability of the colonic commensal bacteria, Bacteroides ovatus, E. coli (SLF) and Lactobacillus rhamnosus (LGG) to modulate production of different cytokines (n = 15) by primary CEC. Antibody staining and flow cytometry were used to investigate Toll-like receptor (TLR) expression by CEC directly ex vivo and TLR responsiveness was determined by examining the ability of TLR ligands to influence CEC cytokine production.
Results: Primary CEC constitutively expressed functional TLR2 and TLR4. Cultured in complete medium alone, CEC secreted IL-6, MCP-1 and IP-10 the levels of which were significantly increased upon addition of the TLR ligands peptidoglycan (PGN) and lipopolysaccharide (LPS). Exposure to the commensal bacteria induced or up-regulated different patterns of cytokine production and secretion. E. coli induced production of MIP-1alpha/beta and betadefensin3 whereas B. ovatus and L. rhamnosus exclusively induced MCP-1 and MIP-2alpha expression, respectively. TNFalpha, RANTES and MEC were induced or up-regulated in response to some but not all of the bacteria whereas ENA78 and IP-10 were up-regulated in response to all bacteria. Evidence of bacterial interference and suppression of cytokine production was obtained from mixed bacterial: CEC co-cultures. Probiotic LGG suppressed E. coli- and B. ovatus-induced cytokine mRNA accumulation and protein secretion.
Conclusion: These observations demonstrate the ability of primary CEC to respond to and discriminate between different strains of commensal bacteria and identify a mechanism by which probiotic bacteria (LGG) may exert anti-inflammatory effects in vivo.
Figures
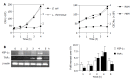
Figure 1
Evaluation of the purity and response of cultured murine CEC. A: CEC from 4-6 wk old C57BL/6 mice were cultured for 72 h in medium alone (M) or in medium containing Bacteroides ovatus (Bo), E. coli (Ec) or Lactobacillus rhamnosus (Lr) after which CEC RNA was extracted, reverse transcribed and cDNA amplified by RT-PCR using primers specific for CD45 or vimentin. PCR products were separated by gel electrophoresis and EtBr-stained amplicons visualized and digitally recorded under UV illumination. The sensitivity of CD45 detection was determined by adding spleen cells to highly purified CECs so that they comprised 2% or 10% of the total cell population prior to RNA extraction and RT-PCR analysis. Control samples (Ctrl) were spleen cells (+Ctrl) and no cDNA (-Ctrl) for CD45 RT-PCR and fibroblasts for vimentin RT-PCR assay. The results are representative of more than 10 independent experiments; B: TLR2 and TLR4 expression by CEC. The dashed line on the histogram plots represents staining with control antibody, the bold line represents staining profile of anti-TLR4 and the filled in histogram plot represents anti-TLR2 antibody staining; C: Responsiveness of TLR expressed by CEC. Supernatants from 4 h cultures of CEC in medium alone (Med) or in medium containing LPS (10 μg/mL) or PGN (1 μg/mL) were assayed for the presence of IL-6 and MCP-1 by ELISA. The results shown were collated from three independent experiments. The error bars represent SEM. a_P_<0.05 LPS vs medium values, b_P_<0.01 PGN vs medium values, c_P_<0.002 LPS vs medium values.
Figure 2
Bacterial growth (A) and kinetics of CEC cytokine gene expression (B) in CEC:bacteria co-cultures. A: Determination of bacterial (L. rhamnosus and E. coli) CFU by harvesting cells from CEC:bacteria co-cultures at hourly intervals up to 4 h by extensive washing of adherent CEC and plating serial dilutions onto agar plates and counting bacterial colonies 24 h later (left-hand panel). B. ovatus were cultured either alone under anaerobic conditions in RGM media or with CEC in 50 mL/L CO2 and complete MEM (right-hand panel). CEC numbers (solid circle on both graphs) were determined by counting the number of cells recovered from co-cultures at the indicated times using a counting chamber; B: CEC cultured in the presence of E. coli for up to 5 h. CECs were processed for RNA isolation and RT-PCR analysis using primers specific for the housekeeping gene β-actin, and MIP-1α and TNFα as described in Materials and Methods. Quantitative densitometry was carried out on EtBr-stained gels and the results from three independent experiments were compiled to produce the data shown. Error bars indicate 95% confidence limits.
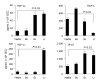
Figure 3
Changes in expression of cytokine genes in CEC in response to commensal bacteria. CECs were cultured for 4 h in complete medium alone (M) or in medium containing Bacteroides ovatus (Bo), E. coli (Ec) or Lactobacillus rhamnosus (Lr) after which RNA was extracted from CECs and processed for RT-PCR analysis using primers for β-actin and genes encoding cytokines and the anti-microbial peptide, β-defensin3 as described in the Materials and methods section. The results shown are typical of those obtained from a total of six independent experiments.
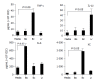
Figure 4
Profile of cytokines secreted by CEC in response to commensal bacteria. CECs were cultured for 4 h in complete medium alone (M) or in medium containing Bacteroides ovatus (Bo), E. coli (Ec) or Lactobacillus rhamnosus (Lr) after which conditioned medium was assayed for the presence of IL-6, TNF-α, IL-1β and KC. The amount of cytokine present was determined by reference to a standard curve generated using known amounts of recombinant protein. The limit of detection of each assay was ~5 pg/mL. The results shown were obtained by combining the data sets from a minimum of three independent experiments. Error bars designate 95% confidence limits.
Figure 5
Chemokines secreted by CEC in response to commensal bacteria. CECs were cultured for 4 h in complete medium alone (M) or in medium containing Bacteroides ovatus (Bo), E. coli (Ec) or Lactobacillus rhamnosus (Lr) after which conditioned medium was assayed for the presence of MIP-1α, MIP-1β, IP-10 and MCP-1. The amount of chemokine present was determined by reference to a standard curve generated using known amounts of recombinant protein. The limit of detection of each assay was ~10 pg/mL. The results shown were obtained by combining the data sets from at least three independent experiments. Error bars designate 95% confidence limits.
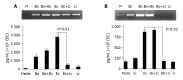
Figure 6
L. rhamnosus (LGG) interferes with E. coli- and _B. ovatus_-induced cytokine production by CEC. CEC were cultured for 4 h with individual bacteria alone or with a mixture of equal numbers of two different bacteria (_B. ovatus_+ E. coli or E. coli+L. rhamnosus) such that the total number of bacteria in each culture was the same. A: KC mRNA and protein expression by RT-PCE and ELISA; B: IL-6 mRNA and protein expression by RT-PCR and ELISA. ELISA data was obtained by combining the data sets from three independent experiments. Error bars designate 95% confidence limits.
References
- Baumgart DC, Dignass AU. Intestinal barrier function. Curr Opin Clin Nutr Metab Care. 2002;5:685–694. - PubMed
- Hecht G. Microbes and microbial toxins: paradigms for microbial-mucosal interactions. VII. Enteropathogenic Escherichia coli: physiological alterations from an extracellular position. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1–G7. - PubMed
- Melmed G, Thomas LS, Lee N, Tesfay SY, Lukasek K, Michelsen KS, Zhou Y, Hu B, Arditi M, Abreu MT. Human intestinal epithelial cells are broadly unresponsive to Toll-like receptor 2-dependent bacterial ligands: implications for host-microbial interactions in the gut. J Immunol. 2003;170:1406–1415. - PubMed
- Fusunyan RD, Nanthakumar NN, Baldeon ME, Walker WA. Evidence for an innate immune response in the immature human intestine: toll-like receptors on fetal enterocytes. Pediatr Res. 2001;49:589–593. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous