Activation of JAK-STAT pathway is required for platelet-derived growth factor-induced proliferation of pancreatic stellate cells - PubMed (original) (raw)
Activation of JAK-STAT pathway is required for platelet-derived growth factor-induced proliferation of pancreatic stellate cells
Atsushi Masamune et al. World J Gastroenterol. 2005.
Abstract
Aim: To clarify the role of Janus kinase-signal transducers and activators of transcription (JAK-STAT) pathway in platelet-derived growth factor (PDGF) induced proliferation in activated pancreatic stellate cells (PSCs).
Methods: PSCs were isolated from rat pancreas tissue, and used in their culture-activated, myofibroblast-like phenotype. STAT-specific binding activity was assessed by electrophoretic mobility shift assay. Activation of Src, JAK2, STAT1, STAT3, and ERK was determined by Western blotting using anti-phospho-specific antibodies. Cell proliferation was assessed by measuring the incorporation of 5-bromo-2'-deoxyuridine.
Results: PDGF-BB induced STAT-specific binding activity, and activation of Src, JAK2, STAT1, STAT3, and ERK. Ethanol and acetaldehyde at clinically relevant concentrations decreased basal activation of JAK2 and STAT3. PDGF-induced activation of STAT1 and STAT3 was inhibited by a Src inhibitor PP1 and a JAK2 inhibitor AG490, whereas PDGF-induced activation of ERK was inhibited by PP1, and not by AG490. PDGF-induced proliferation was inhibited by PP1 and AG490 as well as by STAT3 antisense oligonucleotide.
Conclusion: PDGF-BB activated JAK2-STAT pathway via Src-dependent mechanism. Activation of JAK2-STAT3 pathway, in addition to ERK, may play a role in PDGF-induced proliferation of PSCs.
Figures
Figure 1
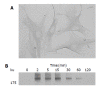
Activated PSCs expressed PDGF β-receptor. A: Serum-starved, culture-activated PSCs were grown directly on slides. Immunostaining for PDGF β-receptor was performed using a streptavidin-biotin-peroxidase complex detection kit. Original magnification: ×20 objective; B: Serum-starved PSCs were treated with PDGF-BB (at 25 ng/mL) for the indicated time. Total cell lysates were prepared, and separated by 70 g/L SDS-polyacrylamide gel electrophoresis. The phosphorylation of PDGF β-receptor was examined by Western blotting.
Figure 2
PDGF activated STAT1 and STAT3. A: PSCs were treated with PDGF-BB (at 25 ng/mL, lane 2) in serum-free medium for 15 min. Nuclear extracts were prepared and subjected to electrophoretic mobility shift assay using STAT consensus oligonucleotide probe m67. Arrow denotes specific inducible complex competitive with cold double-stranded oligonucleotide probe (lane 3). For super shift assays, nuclear extracts were incubated with antibodies against STAT1 (lane 4) or STAT3 (lane 5) before incubation with the radiolabeled probe. *: super shifts. Lane 1: control (serum-free medium only); B: PSCs were treated with PDGF-BB (at 25 ng/mL) for the indicated time. Total cell lysates (approximately 100 μg) were prepared, and separated by 100 g/L SDS-polyacrylamide gel electrophoresis. The activation of STAT1 and STAT3 was examined by Western blotting using anti-phosphospecific antibodies. The levels of total STAT1 and STAT3 were also determined.
Figure 3
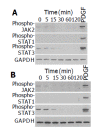
Src and JAK2 mediate the activation of STAT1 and STAT3. A: PSCs were treated with PDGF-BB (at 25 ng/mL) for the indicated time. Total cell lysates (approximately 100 μg) were prepared, and separated by 100 g/L SDS-polyacrylamide gel electrophoresis. The activation of Src and JAK2 was examined by Western blotting using anti-phosphospecific antibodies. The level of GAPDH was also determined; B-D: PSCs were treated with a Src inhibitor PP1 (at 1 or 2.5 μmol/L) or a JAK2 inhibitor AG490 (at 10 or 25 μmol/L) in the absence or presence of PDGF-BB (at 25 ng/mL) for 5 min. Total cell lysates (approximately 100 μg) were prepared, and the levels of phosphorylated JAK2, STAT1, STAT3, and PDGF β-receptor were determined by Western blotting. The levels of total STAT3 and GAPDH were also determined.
Figure 4
Ethanol and acetaldehyde decreased basal activation of JAK2 and STAT3. PSCs were treated with ethanol (at 50 mmol/L, panel A) or acetaldehyde (at 200 mmol/L, panel B) for the indicated time, or with PDGF-BB (at 25 ng/mL) for 5 min. Total cell lysates (approximately 100 μg) were prepared, and separated by 100 g/L SDS-polyacrylamide gel electrophoresis. The activation of JAK2, STAT1, and STAT3 was examined by Western blotting using anti-phosphospecific antibodies. The level of GAPDH was also determined.
Figure 5
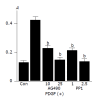
Activation of Src and JAK2 is required for PDGF-induced proliferation and serum-starved PSCs were treated with PDGF-BB (at 25 ng/mL) in the presence or absence of AG490 (at 10 or 25 μmol/L) or PP1 (at 1 or 2.5 μmol/L). After 24-h incubation, DNA synthesis was assessed by BrdU incorporation ELISA. Data are shown as mean±SD (n = 6). b_P_<0.01 versus PDGF-BB only. Con: control (serum-free medium only), A: optical density.
Figure 6
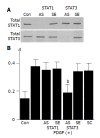
Activation of STAT3, and not STAT1, is required for PDGF-induced proliferation. A: PSCs were treated with antisense (“AS”) or sense (“SE”) oligonucleotides for STAT1 or STAT3 for 24 h. The expression of total STAT1 and STAT3 was examined by Western blotting; B: PSCs were treated with sense, antisense, or scramble (“SC”) oligonucleotides. After 24 h, cells were stimulated with PDGF-BB (at 25 ng/mL) for another 24 h, and DNA synthesis was assessed by BrdU incorporation enzyme-linked immunosorbent assay. Data are shown as mean±SD (% of the control, n = 6). b_P_<0.01 vs PDGF-BB only. Con: control (serum-free medium only), A: optical density.
Figure 7
Src, not JAK2, is located upstream of ERK. PSCs were treated with a JAK2 inhibitor AG490 (A) (at 10 or 25 μmol/L), a Src inhibitor PP1 (B) (at 1 or 2.5 μol/L), or a MAP kinase kinase inhibitor U0126 (C) (at 5 or 10 μmol/L) in the absence or presence of PDGF-BB (at 25 ng/mL) for 5 min. Total cell lysates were prepared, and the levels of phosphorylated ERK, STAT1, and STAT3 were determined by Western blotting. The level of total ERK was also determined.
Figure 8
Signaling pathways for PDGF-induced proliferation in PSCs.
Similar articles
- Role of the JAK-STAT pathway in PDGF-stimulated proliferation of human airway smooth muscle cells.
Simon AR, Takahashi S, Severgnini M, Fanburg BL, Cochran BH. Simon AR, et al. Am J Physiol Lung Cell Mol Physiol. 2002 Jun;282(6):L1296-304. doi: 10.1152/ajplung.00315.2001. Am J Physiol Lung Cell Mol Physiol. 2002. PMID: 12003786 - Cytosolic phospholipase A2 is an effector of Jak/STAT signaling and is involved in platelet-derived growth factor BB-induced growth in vascular smooth muscle cells.
Yellaturu CR, Rao GN. Yellaturu CR, et al. J Biol Chem. 2003 Mar 14;278(11):9986-92. doi: 10.1074/jbc.M211276200. Epub 2003 Jan 15. J Biol Chem. 2003. PMID: 12529382 - Role of the Janus kinase (JAK)/signal transducters and activators of transcription (STAT) cascade in advanced glycation end-product-induced cellular mitogenesis in NRK-49F cells.
Huang JS, Guh JY, Hung WC, Yang ML, Lai YH, Chen HC, Chuang LY. Huang JS, et al. Biochem J. 1999 Aug 15;342 ( Pt 1)(Pt 1):231-8. Biochem J. 1999. PMID: 10432321 Free PMC article. - IL-3 signaling and the role of Src kinases, JAKs and STATs: a covert liaison unveiled.
Reddy EP, Korapati A, Chaturvedi P, Rane S. Reddy EP, et al. Oncogene. 2000 May 15;19(21):2532-47. doi: 10.1038/sj.onc.1203594. Oncogene. 2000. PMID: 10851052 Review. - The Jak-Stat pathway in normal and perturbed hematopoiesis.
Ward AC, Touw I, Yoshimura A. Ward AC, et al. Blood. 2000 Jan 1;95(1):19-29. Blood. 2000. PMID: 10607680 Review. No abstract available.
Cited by
- Tumor microenvironment in chemoresistance, metastasis and immunotherapy of pancreatic cancer.
Wang S, Li Y, Xing C, Ding C, Zhang H, Chen L, You L, Dai M, Zhao Y. Wang S, et al. Am J Cancer Res. 2020 Jul 1;10(7):1937-1953. eCollection 2020. Am J Cancer Res. 2020. PMID: 32774994 Free PMC article. Review. - CCK1 and CCK2 receptors are expressed on pancreatic stellate cells and induce collagen production.
Berna MJ, Seiz O, Nast JF, Benten D, Bläker M, Koch J, Lohse AW, Pace A. Berna MJ, et al. J Biol Chem. 2010 Dec 10;285(50):38905-14. doi: 10.1074/jbc.M110.125534. Epub 2010 Sep 14. J Biol Chem. 2010. PMID: 20843811 Free PMC article. - Nox5 mediates PDGF-induced proliferation in human aortic smooth muscle cells.
Jay DB, Papaharalambus CA, Seidel-Rogol B, Dikalova AE, Lassègue B, Griendling KK. Jay DB, et al. Free Radic Biol Med. 2008 Aug 1;45(3):329-35. doi: 10.1016/j.freeradbiomed.2008.04.024. Epub 2008 Apr 26. Free Radic Biol Med. 2008. PMID: 18466778 Free PMC article. - The incidence, correlation with tumor-infiltrating inflammation, and prognosis of phosphorylated STAT3 expression in human gliomas.
Abou-Ghazal M, Yang DS, Qiao W, Reina-Ortiz C, Wei J, Kong LY, Fuller GN, Hiraoka N, Priebe W, Sawaya R, Heimberger AB. Abou-Ghazal M, et al. Clin Cancer Res. 2008 Dec 15;14(24):8228-35. doi: 10.1158/1078-0432.CCR-08-1329. Clin Cancer Res. 2008. PMID: 19088040 Free PMC article.
References
- Bachem MG, Schneider E, Gross H, Weidenbach H, Schmid RM, Menke A, Siech M, Beger H, Grünert A, Adler G. Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology. 1998;115:421–432. - PubMed
- Masamune A, Kikuta K, Satoh M, Sakai Y, Satoh A, Shimosegawa T. Ligands of peroxisome proliferator-activated receptor-gamma block activation of pancreatic stellate cells. J Biol Chem. 2002;277:141–147. - PubMed
- Masamune A, Sakai Y, Kikuta K, Satoh M, Satoh A, Shimosegawa T. Activated rat pancreatic stellate cells express intercellular adhesion molecule-1 (ICAM-1) in vitro. Pancreas. 2002;25:78–85. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous