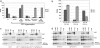Dissociated phenotypes in presenilin transgenic mice define functionally distinct gamma-secretases - PubMed (original) (raw)
. 2005 Jun 21;102(25):8972-7.
doi: 10.1073/pnas.0500940102. Epub 2005 Jun 10.
Paul M Mathews, M Azhar Chishti, Stephen D Schmidt, Yongjun Gu, Jing Yang, Matthew J Mazzella, Janaky Coomaraswamy, Patrick Horne, Bob Strome, Heather Pelly, Georges Levesque, Chris Ebeling, Ying Jiang, Ralph A Nixon, Richard Rozmahel, Paul E Fraser, Peter St George-Hyslop, George A Carlson, David Westaway
Affiliations
- PMID: 15951428
- PMCID: PMC1149500
- DOI: 10.1073/pnas.0500940102
Dissociated phenotypes in presenilin transgenic mice define functionally distinct gamma-secretases
Peter Mastrangelo et al. Proc Natl Acad Sci U S A. 2005.
Abstract
Gamma-secretase depends on presence of presenilins (PS), Nct, Aph-1, and PEN-2 within a core complex. This endoproteolytic activity cleaves within transmembrane domains of amyloid-beta precursor protein (APP) and Notch, and familial Alzheimer's disease (FAD) mutations in PS1 or PS2 genes shift APP cleavage from production of amyloid-beta (Abeta) 40 peptide to greater production of Abeta42. Although studies in PS1/PS2-deficient embryonic cells define overlapping activities for these proteins, in vivo complementation of PS1-deficient animals described here reveals an unexpected spectrum of activities dictated by PS1 and PS2 alleles. Unlike PS1 transgenes, wild-type PS2 transgenes expressed in the mouse CNS support little Abeta40 or Abeta42 production, and FAD PS2 alleles support robust production of only Abeta42. Although wild-type PS2 transgenes failed to rescue Notch-associated skeletal defects in PS1 hypomorphs, a "gained" competence in this regard was apparent for FAD alleles of PS2. The range of discrete and divergent processing activities in mice reconstituted with different PS genes and alleles argues against gamma-secretase being a single enzyme with intrinsically relaxed substrate and cleavage site specificities. Instead, our studies define functionally distinct gamma-secretase variants. We speculate that extrinsic components, in combination with core complexes, may tailor functional variants of this enzyme to their preferred substrates.
Figures
Fig. 1.
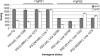
Aβ production in PS2 Tg mice. ELISA analysis of endogenous Aβ species in Tg mouse lines used in this study. TgPS1 mice have been described previously: Tg(L286V)1272 and Tg(PS1wt)1098 mice express similar levels of human PS1 mRNA and NTF, whereas Tg(M146L)line 1 mice express higher levels of mRNA such that PS1 holoprotein can be detected in addition to NTF (10, 45). Numbers of animals in each sample group are, from left to right, n = 5, 4, 3, 4, 5, 4, 6, and 3. Note that all TgPS2 lines have reduced levels of Aβ40 as compared with non-Tg mice but that this situation does not apply to TgPS1 counterparts. For Aβ40 values measured vs. non-Tg mice, results are as follows: PS2 wt line 32790, P = 2.3 × 10–5; PS2 wt line 32799, P = 3.5 × 10–6; PS2 N141I line 1032, P = 3.2 × 10–5; and PS2 M239V line 1379, P = 3 × 10–5. For Aβ40 values measured in Tg PS1 mice vs. non-Tg mice, results were as follows: P = 0.4 for PS1 wt line 1098, P = 0.48 for PS1 L286V line 1272, and P = 0.4 for PS1 M146L line 1. For Aβ42 values measured vs. non-Tg mice, there were significant reductions by wt PS2 transgenes (PS2 wt line 32790, P = 0.00011; PS2 wt line 32799, P = 3.0 × 10–5) and significant elevations by FAD PS2 transgenes (PS2 N141 line 1032, P = 2.4 × 10–4; PS2 M239V line 1379, P = 2.2 × 10–3). For Aβ42 values measured in Tg PS1 mice vs. non-Tg mice, results are as follows: P = 0.11 for PS1wt line 1098, P = 5.5 × 10–6 for PS1 L286V line 1272, and P = 7 × 10–7 for PS1 M146L line 1.
Fig. 2.
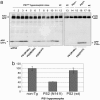
Effects of PS1 and PS2 transgenes on the abnormal skeletal morphology present in PS1-hypomorphs. All animals shown are homozygous for the hypomorphic PS1 allele. Row 1 shows an animal with no superimposed transgene, whereas rows 2–6 represent animals with superimposed transgenes expressing mutant PS1, wtPS2, mutant PS2, or wt APP transgenes (as indicated). The disorganized and reduced number of vertebrae (10) and reduced number of ribs, fusion of ribs (arrow), and aberrant tail morphology present in PS1-null homozygotes is remedied by mutant PS1 and PS2 transgenes (these mice have 13 normal thoracic vertebrae and ribs) but not by a TgAPP695.6209 transgene (46) nor by the wt PS2 transgene (only 10–11 ribs, with the last 4 ribs partially detached).
Fig. 3.
Effects of PS transgenes on APP CTFs. (a) Western blot analysis of APP CTFs. Brain homogenates were electrophoresed on 10–20% tricine gradient gels and blotted with anti-APP C-terminal antibody C1/6.1 (47). Equal loading and transfer of protein was verified by staining the membranes with Ponceau red. Only the superimposed Tg PS1 (M146L) transgenes eliminated the accumulation of APP CTFs found in PS1 hypomorphic mice. (b) Quantitative analysis of APP CTFs that are presented on the left side of a.
Fig. 4.
Aβ production in Tg-complemented PS-deficient mice. Aβ40 and Aβ42 levels were determined by ELISA of adult mouse brain homogenates as described in Materials and Methods. (a) Transgene rescue in mice homozygous for the PS1 hypomorphic alleles. Numbers of animals in each sample group are, from left to right, n = 3, 7, 5, 6, 3, and 5. (b) Transgene rescue in mice homozygous for the PS1-null allele. Five animals were in each sample group. Results are the mean of four measurements ± SE expressed as femtomoles of Aβ per gram of wet brain. The sensitivity of the Aβ sandwich ELISAs was 38.7 and 18.9 fmol/g analyzed for Aβ40 and Aβ42, respectively. The Aβ40-sensitivity threshold is indicated by a horizontal dotted line drawn across the bottom of each of the bar graphs. For comparisons in a of non-Tg vs. PS1(M146L), PS2wt, PS2(N141I), and PS2 (M239V) for Aβ40, P = 8 × 10–10, 0.02, 0.008, and 0.01, respectively, and for Aβ42, P = 4 × 10–7, 0.37, 10–4, and 1.8 × 10–11, respectively. (c) A 2D gel analysis of γ-secretase component proteins from mouse brain. Shown are analyses in PS1-deficient mice either heterozygous (Left) or homozygous (Center and Right) for the fully penetrant null allele. The genotypes of superimposed PS1 or PS2 transgenes are indicated. Native electrophoresis is represented in the horizontal dimension, and size markers (arrows) are indicated in kDa. Denaturing electrophoresis in the presence of SDS is shown in the vertical dimension, and size markers for the corresponding blots are, from top to bottom, 100, 45, 28, and 23 kDa (arrowheads). Antibodies used to probe the resultant blots are shown on the right side. A nonspecific band detected with the Aph-1 antibody is indicated with an asterisk. In addition to 28- to 30-kDa signals seen for PS1 or PS2 NTFs, the faint signals seen at higher molecular masses correspond to uncleaved holoprotein. (d) Analyses in Tg mice homozygous wt for the endogenous PS1 gene. Note the population of lower _M_r complexes containing PS2-NTF (center to right side of PS2 blot) vs. analogous PS1-NTF signals for complexes containing either endogenous mouse PS1 or transgene-encoded human PS1.
Similar articles
- Genes and mechanisms involved in beta-amyloid generation and Alzheimer's disease.
Steiner H, Capell A, Leimer U, Haass C. Steiner H, et al. Eur Arch Psychiatry Clin Neurosci. 1999;249(6):266-70. doi: 10.1007/s004060050098. Eur Arch Psychiatry Clin Neurosci. 1999. PMID: 10653281 Review. - Mutant presenilins specifically elevate the levels of the 42 residue beta-amyloid peptide in vivo: evidence for augmentation of a 42-specific gamma secretase.
Jankowsky JL, Fadale DJ, Anderson J, Xu GM, Gonzales V, Jenkins NA, Copeland NG, Lee MK, Younkin LH, Wagner SL, Younkin SG, Borchelt DR. Jankowsky JL, et al. Hum Mol Genet. 2004 Jan 15;13(2):159-70. doi: 10.1093/hmg/ddh019. Epub 2003 Nov 25. Hum Mol Genet. 2004. PMID: 14645205 - Characterization of the reconstituted gamma-secretase complex from Sf9 cells co-expressing presenilin 1, nicastrin [correction of nacastrin], aph-1a, and pen-2.
Zhang L, Lee J, Song L, Sun X, Shen J, Terracina G, Parker EM. Zhang L, et al. Biochemistry. 2005 Mar 22;44(11):4450-7. doi: 10.1021/bi0481500. Biochemistry. 2005. PMID: 15766275 - Presenilin clinical mutations can affect gamma-secretase activity by different mechanisms.
Bentahir M, Nyabi O, Verhamme J, Tolia A, Horré K, Wiltfang J, Esselmann H, De Strooper B. Bentahir M, et al. J Neurochem. 2006 Feb;96(3):732-42. doi: 10.1111/j.1471-4159.2005.03578.x. Epub 2006 Jan 9. J Neurochem. 2006. PMID: 16405513 - Role of presenilin in gamma-secretase cleavage of amyloid precursor protein.
Xia W. Xia W. Exp Gerontol. 2000 Jul;35(4):453-60. doi: 10.1016/s0531-5565(00)00111-x. Exp Gerontol. 2000. PMID: 10959033 Review.
Cited by
- From soluble aβ to progressive aβ aggregation: could prion-like templated misfolding play a role?
Eisele YS. Eisele YS. Brain Pathol. 2013 May;23(3):333-41. doi: 10.1111/bpa.12049. Brain Pathol. 2013. PMID: 23587139 Free PMC article. Review. - Assembly, maturation, and trafficking of the gamma-secretase complex in Alzheimer's disease.
Dries DR, Yu G. Dries DR, et al. Curr Alzheimer Res. 2008 Apr;5(2):132-46. doi: 10.2174/156720508783954695. Curr Alzheimer Res. 2008. PMID: 18393798 Free PMC article. Review. - Presenilin-1 processing of ErbB4 in fetal type II cells is necessary for control of fetal lung maturation.
Hoeing K, Zscheppang K, Mujahid S, Murray S, Volpe MV, Dammann CE, Nielsen HC. Hoeing K, et al. Biochim Biophys Acta. 2011 Mar;1813(3):480-91. doi: 10.1016/j.bbamcr.2010.12.017. Epub 2010 Dec 29. Biochim Biophys Acta. 2011. PMID: 21195117 Free PMC article. - A pathogenic PSEN2 p.His169Asn mutation associated with early-onset Alzheimer's disease.
Giau VV, Pyun JM, Bagyinszky E, An SSA, Kim S. Giau VV, et al. Clin Interv Aging. 2018 Jul 31;13:1321-1329. doi: 10.2147/CIA.S170374. eCollection 2018. Clin Interv Aging. 2018. PMID: 30104866 Free PMC article. - In-Frame and Frameshift Mutations in Zebrafish Presenilin 2 Affect Different Cellular Functions in Young Adult Brains.
Barthelson K, Pederson SM, Newman M, Jiang H, Lardelli M. Barthelson K, et al. J Alzheimers Dis Rep. 2021 May 4;5(1):395-404. doi: 10.3233/ADR-200279. J Alzheimers Dis Rep. 2021. PMID: 34189411 Free PMC article.
References
- Wong, P. C., Zheng, H., Chen, H., Becher, M., Sirinathsinghji, D. J. S., Trumbauer, M. E., Chen, H. Y., Price, D. L., Van der Ploeg, L. H. T. & Sisodia, S. S. (1997) Nature 387, 288–292. - PubMed
- De Strooper, B., Saftig, P., Craessaerts, K., Vanderstichele, H., Guhde, G., Annaert, W., Von Figura, K. & Van Leuven, F. (1998) Nature 391, 387–390. - PubMed
- Schroeter, E. H., Kisslinger, J. A. & Kopan, R. (1998) Nature 393, 382–386. - PubMed
- Cao, X. & Sudhof, T. C. (2001) Science 293, 115–120. - PubMed
- Yu, G., Chen, F., Levesque, G., Nishimura, M., Zhang, D. M., Levesque, L., Rogaeva, E., Xu, D., Liang, Y., Duthie, M., et al. (1998) J. Biol. Chem. 273, 16470–16475. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases