In vivo imaging of colitis and colon cancer development in mice using high resolution chromoendoscopy - PubMed (original) (raw)
In vivo imaging of colitis and colon cancer development in mice using high resolution chromoendoscopy
C Becker et al. Gut. 2005 Jul.
Abstract
Background: Mouse models of colitis and cancer are indispensable for our understanding of the pathogenesis of these diseases. In the past, mice had to be sacrificed in order to analyse colitis activity and tumour development. We have developed a safe method for high resolution endoscopic monitoring of living mice.
Methods: Mice developing colitis or colonic tumours were anaesthetised using avertine and repeatedly examined by endoscopy. A novel miniendoscope (1.9 mm outer diameter), denoted Coloview, was introduced via the anus and the colon was carefully insufflated with an air pump before analysis of the colonic mucosa. An extra working channel allowed the introduction of biopsy forceps or injection needles as well as surface staining with methylene blue in order to visualise the surface of the crypts and the pit pattern architecture.
Results: Endoscopic pictures obtained were of high quality and allowed monitoring and grading of disease. Scoring of colitis activity as well as tumour size and growth was possible. In addition, pit pattern analysis using chromoendoscopy permitted discrimination between inflammatory and neoplastic changes. Biopsies yielded enough tissue for molecular and histopathological analyses.
Conclusions: In summary, chromoendoscopy in mice allows monitoring of the development of colitis and colon cancer with high resolution. Manipulations such as local injection of reagents or taking biopsies can be performed easily.
Figures
Figure 1
Experimental setup of the Coloview miniendoscopic system. (A) Schematic diagram of the endoscopic setup. Endoscopic tools used for mouse examinations: straightforward telescope, examination sheath, manipulation sheath, biopsy forceps, and injection tube. (B) Experimental procedure used to induce colon carcinogenesis in FVB mice. Mice were injected intraperitoneally with a single dose (7.4 mg/kg) of the mutagenic agent azoxymethane (AOM) followed by three cycles of dextran sodium sulphate (DSS) in drinking water for one week and normal drinking water for two weeks. E = endoscopic examination.
Figure 2
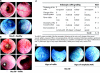
In vivo high resolution endoscopy and chromoendoscopy of mice treated with azoxymethane and dextran sodium sulphate. Mice were anaesthetised by intraperitoneal injection of avertine. The colon mucosa was stained with methylene blue to visualise the crypt pattern. (A) Representative endoscopic pictures showing the colon of a healthy mouse. Note the smooth and transparent mucosa, normal vascular pattern, and regular crypt pattern in the methylene blue stained colon. (B) Representative endoscopic pictures showing signs of severe inflammation. Note the close up pictures of bleeding mucosa, altered vascular pattern, transparent mucosa, and fibrin. (C) Endoscopic colitis score based on the observed signs of inflammation. The modified murine endoscopic index of colitis severity (MEICS) consisted of five parameters, as indicated. (D) Methylene blue staining of the colonic mucosa during endoscopy at day 20. Shown are representative pictures of chromoendoscopic signs of inflammation and early neoplasias (aberrant crypt foci (ACF)).
Figure 3
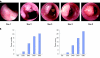
Endoscopic scoring of tumour development in mice. (A) Representative endoscopic pictures showing the development of colon tumours during the course of the experiment. The grading of tumour size was performed as indicated in materials and methods. (B) Number of lesions in each mouse was counted and compared during the course of the experiment (left panel). Alternatively, the size of each tumour was graded at the indicated time points and summed up for each mouse (right panel).
Figure 4
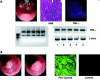
Molecular investigation and manipulation of tumour development in mice. (A) Biopsy sampling (upper left panel) of a tumour during routine endoscopic monitoring of azoxymethane/dextran sodium sulphate treated animals. Tumour biopsies were snap frozen in liquid nitrogen and cut for staining. Subsequent haematoxylin-eosin staining (upper middle panel) demonstrated high grade intraepithelial neoplasias. Immunohistochemistry for tumour necrosis factor α (TNF-α) (upper right panel) of cross sections was performed using the TSA Cy3 system. Cryosections were fixed in acetone. Slides were then incubated with primary antibodies specific for TNF-α. Before examination, nuclei were counterstained with Hoechst 3342. Lower left panel: gel electrophoretic picture showing the high quality of RNA extracted from five tumour biopsies. Tumours typically yielded 1–2 µg RNA per biopsy. Lower right panel: reverse transcription-polymerase chain reaction analysis for TNF-α expressed in tumour biopsies. β-Actin served as a control. (B) Injection of fluorescein isothiocyanate (FITC) into a tumour during endoscopy. A needle mounted onto a thin tube was introduced through the instrument channel of the endoscope and FITC was slowly injected. Tumour samples were then analysed by immunofluorescence. One representative picture of a tumour injected with 20 µl of an FITC solution and a control tumour not injected is shown.
Similar articles
- High resolution colonoscopy in live mice.
Becker C, Fantini MC, Neurath MF. Becker C, et al. Nat Protoc. 2006;1(6):2900-4. doi: 10.1038/nprot.2006.446. Nat Protoc. 2006. PMID: 17406549 - Colonoscopy in mice.
Huang EH, Carter JJ, Whelan RL, Liu YH, Rosenberg JO, Rotterdam H, Schmidt AM, Stern DM, Forde KA. Huang EH, et al. Surg Endosc. 2002 Jan;16(1):22-4. doi: 10.1007/s004640080168. Epub 2001 Oct 13. Surg Endosc. 2002. PMID: 11961598 - Assessment of tumor development and wound healing using endoscopic techniques in mice.
Neurath MF, Wittkopf N, Wlodarski A, Waldner M, Neufert C, Wirtz S, Günther C, Becker C. Neurath MF, et al. Gastroenterology. 2010 Dec;139(6):1837-1843.e1. doi: 10.1053/j.gastro.2010.10.007. Epub 2010 Oct 16. Gastroenterology. 2010. PMID: 20955705 - Dextran sulfate sodium-induced colitis-associated neoplasia: a promising model for the development of chemopreventive interventions.
Clapper ML, Cooper HS, Chang WC. Clapper ML, et al. Acta Pharmacol Sin. 2007 Sep;28(9):1450-9. doi: 10.1111/j.1745-7254.2007.00695.x. Acta Pharmacol Sin. 2007. PMID: 17723178 Review. - Chromoscopic endomicroscopy: in vivo cellular resolution imaging of the colorectum.
Smith LA, Tiffin N, Thomson M, Cross SS, Hurlstone DP. Smith LA, et al. J Gastroenterol Hepatol. 2008 Jul;23(7 Pt 1):1009-23. doi: 10.1111/j.1440-1746.2008.05463.x. Epub 2008 Jun 28. J Gastroenterol Hepatol. 2008. PMID: 18557799 Review.
Cited by
- Target-Specific Fluorescence-Mediated Tomography for Non-Invasive and Dynamic Assessment of Early Neutrophil Infiltration in Murine Experimental Colitis.
Nowacki TM, Lenz P, Bettenworth D, Brückner M, Bokemeyer A, Tepasse PR, Helfen A, Wildgruber M, Eisenblätter M. Nowacki TM, et al. Cells. 2019 Oct 28;8(11):1328. doi: 10.3390/cells8111328. Cells. 2019. PMID: 31661876 Free PMC article. - A Macromolecular Janus Kinase (JAK) Inhibitor Prodrug Effectively Ameliorates Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice.
Zhao G, Wei X, Wu J, Eichele DD, Lele SM, Yang L, Zhang F, Wang D. Zhao G, et al. Pharm Res. 2019 Mar 11;36(4):64. doi: 10.1007/s11095-019-2587-6. Pharm Res. 2019. PMID: 30859327 Free PMC article. - Thymidylate synthase O-GlcNAcylation: a molecular mechanism of 5-FU sensitization in colorectal cancer.
Very N, Hardivillé S, Decourcelle A, Thévenet J, Djouina M, Page A, Vergoten G, Schulz C, Kerr-Conte J, Lefebvre T, Dehennaut V, El Yazidi-Belkoura I. Very N, et al. Oncogene. 2022 Jan;41(5):745-756. doi: 10.1038/s41388-021-02121-9. Epub 2021 Nov 29. Oncogene. 2022. PMID: 34845374 - Topical Therapy with Antisense Tumor Necrosis Factor Alpha Using Novel β-Glucan-Based Drug Delivery System Ameliorates Intestinal Inflammation.
Sakisaka H, Takedatsu H, Mitsuyama K, Mochizuki S, Sakurai K, Sakisaka S, Hirai F. Sakisaka H, et al. Int J Mol Sci. 2020 Jan 20;21(2):683. doi: 10.3390/ijms21020683. Int J Mol Sci. 2020. PMID: 31968666 Free PMC article. - Affective state determination in a mouse model of colitis-associated colorectal cancer.
Chartier LC, Hebart ML, Howarth GS, Whittaker AL, Mashtoub S. Chartier LC, et al. PLoS One. 2020 Jan 27;15(1):e0228413. doi: 10.1371/journal.pone.0228413. eCollection 2020. PLoS One. 2020. PMID: 31986185 Free PMC article.
References
- Wirtz S, Neurath MF. Animal models of intestinal inflammation: new insights into the molecular pathogenesis and immunotherapy of inflammatory bowel disease. Int J Colorectal Dis 2000;15:144–60. - PubMed
- Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol 2002;20:495–549. - PubMed
- Wirtz S, Becker C, Blumberg R, et al. Treatment of T cell-dependent experimental colitis in SCID mice by local administration of an adenovirus expressing IL-18 antisense mRNA. J Immunol 2002;168:411–20. - PubMed
- Huang EH, Carter JJ, Whelan RL, et al. Colonoscopy in mice. Surg Endosc 2002;16:22–4. - PubMed
- Greten FR, Eckmann L, Greten TF, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 2004;118:285–96. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous