Myelin-associated glycoprotein and complementary axonal ligands, gangliosides, mediate axon stability in the CNS and PNS: neuropathology and behavioral deficits in single- and double-null mice - PubMed (original) (raw)
Comparative Study
Myelin-associated glycoprotein and complementary axonal ligands, gangliosides, mediate axon stability in the CNS and PNS: neuropathology and behavioral deficits in single- and double-null mice
Baohan Pan et al. Exp Neurol. 2005 Sep.
Abstract
Complementary interacting molecules on myelin and axons are required for long-term axon-myelin stability. Their disruption results in axon degeneration, contributing to the pathogenesis of demyelinating diseases. Myelin-associated glycoprotein (MAG), a minor constituent of central and peripheral nervous system myelin, is a member of the Siglec family of sialic acid-binding lectins and binds to gangliosides GD1a and GT1b, prominent molecules on the axon surface. Mice lacking the ganglioside biosynthetic gene Galgt1 fail to express complex gangliosides, including GD1a and GT1b. In the current studies, CNS and PNS histopathology and behavior of Mag-null, Galgt1-null, and double-null mice were compared on the same mouse strain background. When back-crossed to >99% C57BL/6 strain purity, Mag-null mice demonstrated marked CNS, as well as PNS, axon degeneration, in contrast to prior findings using mice of mixed strain background. On the same background, Mag- and Galgt1-null mice exhibited quantitatively and qualitatively similar CNS and PNS axon degeneration and nearly identical decreases in axon diameter and neurofilament spacing. Double-null mice had qualitatively similar changes. Consistent with these findings, Mag- and Galgt1-null mice had similar motor behavioral deficits, with double-null mice only modestly more impaired. Despite their motor deficits, Mag- and Galgt1-null mice demonstrated hyperactivity, with spontaneous locomotor activity significantly above that of wild type mice. These data demonstrate that MAG and complex gangliosides contribute to axon stability in both the CNS and PNS. Similar neuropathological and behavioral deficits in Galgt1-, Mag-, and double-null mice support the hypothesis that MAG binding to gangliosides contributes to long-term axon-myelin stability.
Figures
Fig. 1
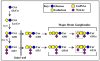
Biosynthesis of major brain gangliosides. The relationships between major brain gangliosides and their precursors are shown schematically, along with the ganglioside nomenclature of Svennerholm (Svennerholm, 1994). The block in ganglioside biosynthesis due to disruption of the Galgt1 gene (Liu et al., 1999) is indicated by a vertical double line (the genes responsible for synthesis of GM3 and GD3, Siat9 and Siat8a, respectively, are also indicated). MAG ligands GD1a and GT1b appear at the right, with the key MAG-binding determinant (NeuAc α2–3 Gal β1–3 GalNAc) indicated with a dotted line.
Fig. 2
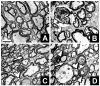
Neuropathology in sciatic nerve axons of _Mag_-, _Galgt1_- and double-null mice. Light microscopic toluidine blue-stained 1-μm Epon cross sections of sciatic nerve. Myelinated fibers undergoing axonal degeneration (arrowheads) and tomacula (arrows) in _Galgt1_-null (B), _Mag_-null (C) and double-null (D) nerves compared with wild type nerves (A). Bar = 10 μm.
Fig. 3
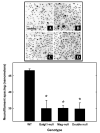
Neuropathology in cervical spinal cords of _Mag_-, _Galgt1_- and double-null mice. Low magnification electron microscopic images of transverse sections of the dorsal funiculus of wild type (A), _Galgt1_-null (B), _Mag_-null (C), and double-null (D) mice. Wallerian degeneration (arrowheads), dysmyelinated axons (arrows) and axon swelling (asterisk) are noted. Bar = 2 μm.
Fig. 4
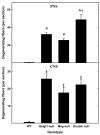
Increased axon degeneration in _Mag_-, _Galgt1_- and double-null mice. Sciatic nerve (PNS, top panel) and spinal dorsal column (CNS, bottom panel) cross sections were viewed for evidence of degenerating axons in the following mice (number, average age): _Mag_-null (n=6, 6.3 months), _Galgt1_-null (n=5, 7.4 months), double-null (n=6, 7.9 months), and wild type C57BL/6 (n=6, 6 months). Data are presented as means ± SE. One-way ANOVA for the number of degenerating axons revealed highly significant differences based on genotype (p < 0.01) for both PNS and CNS. Post-hoc analysis (Fisher’s PLSD) revealed significant increases (*, p < 0.0001) in the number of degenerating PNS axons in _Galgt1_-, _Mag_- and double-null mice compared to wild type mice, and significantly more degenerating axons in the double-null mice compared to the single-null mice (†, p < 0.01). Post-hoc analysis of the CNS data indicated highly significant increases in degenerating axons (§, p < 0.01) in all mutant strains compared to wild type, but no difference among the mutant strains.
Fig. 5
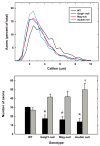
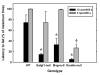
Decreased axon caliber in sciatic nerves of _Mag_-, _Galgt1_- and double-null mice. Micrographs of sciatic nerve cross sections were subjected to image analysis to determine the diameters of myelinated axons from the following mice (average age): _Mag_-null (6.2 months) _Galgt1_-null (7.4 months), double-null (6.3 months), and wild type (6 months). Diameters of ~500 axons each from 3 mice of each genotype were determined. Top panel: Histograms of pooled axon diameters for each genotype. Bottom panel: Combined data representing the percent of axons ≥5 μm diameter (black bars) and ≤2.5 μm diameter (gray bars). The data are presented as mean ± SE (n = 3). One-way ANOVA for the number of axons >5 μm diameter revealed a significant difference based on genotype (p < 0.05). Post-hoc analysis (Fisher’s PLSD) revealed significant decreases (*) in axon caliber for _Galgt1_-null mice (p < 0.05), _Mag_-null mice (p < 0.05) and double-null mice (p < 0.01) compared to wild type mice. Although_Galgt1_-null and _Mag_-null mice demonstrated a trend toward greater numbers of axons ≤2.5 μm diameter (p = 0.08), only double-null mice expressed significantly more of these smaller myelinated axons (†, p < 0.05).
Fig. 6
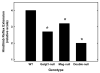
Decreased neurofilament spacing in sciatic nerve axons of _Mag_-, _Galgt1_- and double-null mice. Top panel: Electron micrographic images of cross sections of sciatic nerves, showing individual neurofilaments and their spacing, in wild type (A), _Galgt1_-null (B), _Mag_-null (C), and double-null (D) mice. Bar = 0.2 μm. Bottom panel: Nearest neighbor spacing was determined for ≥15 axons from each of three mice of each genotype (age): _Mag_-null (6.2 months), _Galgt1_-null (7.4 months), double-null (6.3 months), and wild type C57BL/6 (6 months). Values are presented as mean ± SE. One-way ANOVA for neurofilament spacing was highly significant (p < 0.01). Post-hoc analysis (Fisher’s PLSD) revealed that neurofilaments of _Galgt1_-, _Mag_- and double-null mice had significantly reduced spacing compared to wild type mice (*, p ≤ 0.001).
Fig. 7
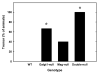
Impaired motor coordination and balance in _Mag_-null, _Galgt1_-null and double-null mice. Motor coordination was evaluated by measuring the latency to fall using a rotarod apparatus (5 cm diameter drum) at two fixed speeds, 10 rpm (black bars, maximum 600s), and 5 rpm (gray bars, maximum 300 s). Data are presented as mean ± SE. Mice tested included (number, average age): _Mag_-null (n=5, 5.4 months); _Galgt1_-null (n=3, 6.9 months); double-null (n=5, 4.7 months); and wild type C57BL/6 (n=4, 6 months). At 10 rpm, one-way ANOVA for latency to fall was highly significant (p < 0.001). Post-hoc analysis (Fisher’s PLSD) revealed that _Galgt1_-null mice (p < 0.01), _Mag_-null mice (p < 0.01) and double-null mice (p < 0.001) were significantly impaired (*) compared to wild type mice, and that double-null mice were significantly impaired (p < 0.05) compared to _Mag_-null mice. Under less stringent conditions (5 rpm) one-way ANOVA for latency to fall was also highly significant (p < 0.001), although post-hoc analysis revealed that only double-null mice were significantly impaired in comparison to each of the other genotypes (†, p ≤ 0.001).
Fig. 8
Impaired hindlimb reflexes in _Mag_-null, _Galgt1_-null and double-null mice. Wild type mice typically hold their hindlimbs extended steadily at an angle of ≥90° when lifted by the tail. The position of the hindlimbs of wild type and mutant mice were scored on a scale from 0 (most impaired, no reflex) to 4 (normal). The best performance in three trials was averaged for 3–5 mice of each genotype. Data were analyzed using the Kruskal-Wallis nonparametric test and are shown as means for clarity. There was a significant effect of genotype (*, p < 0.01) with all mutant strains impaired compared to wild type mice. Ages and numbers of mice used were as detailed in Fig. 7, legend. [Note: non-parametric statistical analysis was used, precluding the use of error bars.]
Fig. 9
Tremor in Mag/Galgt1 double-null mice. All double-null mice displayed severe episodes of body tremor while resting and during movement, whereas no wild type mice displayed tremor under any circumstance. To quantify this observation, whole body tremor was scored (as present or absent) in wild type, _Mag_-null, _Galgt1_-null and double-null mice. Data were analyzed using logistic regression and are shown as a percentage of mice exhibiting tremor for each genotype (n = 3–5). There was a significant effect of genotype with_Galgt1_-null and double-null mice exhibiting significantly more tremor than wild type mice (*, p < 0.05 and p < 0.001 respectively). Although tremor was recorded in a portion of the single-null mice, tremor was a consistent behavior in double-nulls. Ages and numbers of mice used were as detailed in Fig. 7, legend. [Note: non-parametric statistical analysis was used, precluding the use of error bars].
Fig. 10
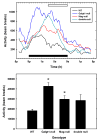
Hyperactivity in _Mag_-null, _Galgt1_-null and double-null mice. Spontaneous locomotor activity was determined for 3–6 individual mice of each genotype in two 24-h sessions in 29 x 50 cm Plexiglas boxes, each fitted with a grid of infrared beams. Top panel: The number of beam breaks per interval was averaged for mice of each genotype as a function of time. Data were smoothed over five data points (100 min). The solid bar designates the 12-h dark cycle. Over the time period constituting the latter half of the dark cycle (open bar), each mutant mouse strain was significantly more active than wild type mice (_Mag_-null, p < 0.01; _Galgt1_-null, p < 0.001; double-null, p < 0.03, pairwise Student’s t-tests). Bottom panel: The average total beam breaks over the entire 24-h test period was determined for mice of each genotype. Data are presented as mean ± SE. One-way ANOVA for total activity was highly significant (p < 0.01). Post-hoc analysis (Fisher’s PLSD) revealed that both _Galgt1_-null mice (p < 0.001) and_Mag_-null mice (p < 0.05) were significantly more active (*) than wild type mice over the entire test period, with double-null mice trending in the same direction (p = 0.06). The following mice were used (number, average age): _Mag_-null (n=6, 5.4 months); _Galgt1_-null (n=3, 6.9 months); double-null (n=5, 4.9 months); and wild type C57BL/6 (n=4, 6.5 months).
Similar articles
- Myelin-associated glycoprotein (Siglec-4) expression is progressively and selectively decreased in the brains of mice lacking complex gangliosides.
Sun J, Shaper NL, Itonori S, Heffer-Lauc M, Sheikh KA, Schnaar RL. Sun J, et al. Glycobiology. 2004 Sep;14(9):851-7. doi: 10.1093/glycob/cwh107. Epub 2004 Jun 2. Glycobiology. 2004. PMID: 15175257 - Brain gangliosides: functional ligands for myelin stability and the control of nerve regeneration.
Vyas AA, Schnaar RL. Vyas AA, et al. Biochimie. 2001 Jul;83(7):677-82. doi: 10.1016/s0300-9084(01)01308-6. Biochimie. 2001. PMID: 11522397 Review. - Brain gangliosides in axon-myelin stability and axon regeneration.
Schnaar RL. Schnaar RL. FEBS Lett. 2010 May 3;584(9):1741-7. doi: 10.1016/j.febslet.2009.10.011. Epub 2009 Oct 12. FEBS Lett. 2010. PMID: 19822144 Free PMC article. Review. - Gangliosides are neuronal ligands for myelin-associated glycoprotein.
Yang LJ, Zeller CB, Shaper NL, Kiso M, Hasegawa A, Shapiro RE, Schnaar RL. Yang LJ, et al. Proc Natl Acad Sci U S A. 1996 Jan 23;93(2):814-8. doi: 10.1073/pnas.93.2.814. Proc Natl Acad Sci U S A. 1996. PMID: 8570640 Free PMC article. - Glial Sulfatides and Neuronal Complex Gangliosides Are Functionally Interdependent in Maintaining Myelinating Axon Integrity.
McGonigal R, Barrie JA, Yao D, McLaughlin M, Cunningham ME, Rowan EG, Willison HJ. McGonigal R, et al. J Neurosci. 2019 Jan 2;39(1):63-77. doi: 10.1523/JNEUROSCI.2095-18.2018. Epub 2018 Nov 16. J Neurosci. 2019. PMID: 30446529 Free PMC article.
Cited by
- The Axon-Myelin Unit in Development and Degenerative Disease.
Stassart RM, Möbius W, Nave KA, Edgar JM. Stassart RM, et al. Front Neurosci. 2018 Jul 11;12:467. doi: 10.3389/fnins.2018.00467. eCollection 2018. Front Neurosci. 2018. PMID: 30050403 Free PMC article. Review. - Complexity of Generating Mouse Models to Study the Upper Motor Neurons: Let Us Shift Focus from Mice to Neurons.
Genc B, Gozutok O, Ozdinler PH. Genc B, et al. Int J Mol Sci. 2019 Aug 7;20(16):3848. doi: 10.3390/ijms20163848. Int J Mol Sci. 2019. PMID: 31394733 Free PMC article. Review. - Lipid Rafts: The Maestros of Normal Brain Development.
Viljetić B, Blažetić S, Labak I, Ivić V, Zjalić M, Heffer M, Balog M. Viljetić B, et al. Biomolecules. 2024 Mar 18;14(3):362. doi: 10.3390/biom14030362. Biomolecules. 2024. PMID: 38540780 Free PMC article. Review. - Oligodendrocyte development and myelin biogenesis: parsing out the roles of glycosphingolipids.
Jackman N, Ishii A, Bansal R. Jackman N, et al. Physiology (Bethesda). 2009 Oct;24:290-7. doi: 10.1152/physiol.00016.2009. Physiology (Bethesda). 2009. PMID: 19815855 Free PMC article. Review. - Gangliosides in cell recognition and membrane protein regulation.
Lopez PH, Schnaar RL. Lopez PH, et al. Curr Opin Struct Biol. 2009 Oct;19(5):549-57. doi: 10.1016/j.sbi.2009.06.001. Epub 2009 Jul 14. Curr Opin Struct Biol. 2009. PMID: 19608407 Free PMC article. Review.
References
- Bartsch S, Montag D, Schachner M, Bartsch U. Increased number of unmyelinated axons in optic nerves of adult mice deficient in the myelin-associated glycoprotein (MAG) Brain Res. 1997;762:231–234. - PubMed
- Bjartmar C, Kidd G, Mork S, Rudick R, Trapp BD. Neurological disability correlates with spinal cord axonal loss and reduced N-acetyl aspartate in chronic multiple sclerosis patients. Ann Neurol. 2000;48:893–901. - PubMed
- Bjartmar C, Wujek JR, Trapp BD. Axonal loss in the pathology of MS: consequences for understanding the progressive phase of the disease. J Neurol Sci. 2003;206:165–171. - PubMed
- Bjartmar C, Yin X, Trapp BD. Axonal pathology in myelin disorders. J Neurocytol. 1999;28:383–395. - PubMed
- Bruck W, Stadelmann C. Inflammation and degeneration in multiple sclerosis. Neurol Sci. 2003;24:S265–S267. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS042888/NS/NINDS NIH HHS/United States
- NS37096/NS/NINDS NIH HHS/United States
- NS42888/NS/NINDS NIH HHS/United States
- R37 NS037096/NS/NINDS NIH HHS/United States
- R01 NS037096/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials