The exocyst component Sec5 is present on endocytic vesicles in the oocyte of Drosophila melanogaster - PubMed (original) (raw)
The exocyst component Sec5 is present on endocytic vesicles in the oocyte of Drosophila melanogaster
Bernhard Sommer et al. J Cell Biol. 2005.
Abstract
The exocyst is an octameric complex required for polarized secretion. Some components of the exocyst are found on the plasma membrane, whereas others are recruited to Golgi membranes, suggesting that exocyst assembly tethers vesicles to their site of fusion. We have found that in Drosophila melanogaster oocytes the majority of the exocyst component Sec5 is unexpectedly present in clathrin-coated pits and vesicles at the plasma membrane. In oocytes, the major substrate for clathrin-dependent endocytosis is the vitellogenin receptor Yolkless. A truncation mutant of Sec5 (sec5(E13)) allows the formation of normally sized oocytes but with greatly reduced yolk uptake. We find that in sec5(E13) oocytes Yolkless accumulates aberrantly in late endocytic compartments, indicating a defect in the endocytic cycling of the receptor. An analogous truncation of the yeast SEC5 gene results in normal secretion but a temperature-sensitive defect in endocytic recycling. Thus, the exocyst may act in both Golgi to plasma membrane traffic and endocytic cycling, and hence in oocytes is recruited to clathrin-coated pits to facilitate the rapid recycling of Yolkless.
Figures
Figure 1.
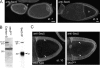
Characterization of an anti-Sec5 antiserum. (A) Confocal micrographs of wild-type ovaries labeled with the rabbit anti-Sec5 antibody. Sec5 is enriched in the oocyte (o) and from around stage 7 (st. 7) becomes localized to the plasma membrane and remains plasma membrane localized throughout vitellogenesis from stages 8–10. Bars, 20 μm. (B) Anti-Sec5 protein blots of total proteins prepared from wild-type (Canton-S) and sec5 E13 eggs (left), or of an anti-Sec5 immunoprecipitation (IP; right) from wild-type ovary extract. The antibody recognizes two prominent bands, one of which is specific for native Sec5, whose predicted molecular mass is 100.7 kD. (C) As A, except anti-Sec5 was applied to stage 10 oocytes from wild-type or sec5 E13 germline clones. Anti-Sec5 staining along the plasma membrane of the wild-type oocyte is indicated by arrows and is absent in the sec5 E13 germline clone. The somatically derived border cells are positive for Sec5 in both wild type and mutant.
Figure 2.
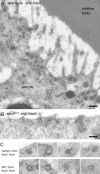
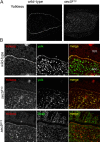
Sec5 localizes to clathrin-coated pits and vesicles underneath the plasma membrane of Drosophila oocytes. (A and B) Electron micrographs of cryosections of a wild-type oocyte or of an oocyte from a sec5 E13 germline labeled with anti-Sec5 followed by protein A conjugated to 10-nm gold particles. The gold particles decorate structures with electron-dense coats, and such labeling is absent from the section from sec5 E13. Bars, 200 nm. (C) Electron micrographs as in A of coated pits and vesicles labeled for Sec5, clathrin, and α-adaptin (AP2) as indicated.
Figure 3.
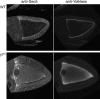
Sec5 plasma membrane labeling is reduced in _yl_15 mutant oocytes. (A) Confocal micrographs of oocytes from wild type (WT; Canton-S) or homozygous yl 15 females labeled for both Sec5 and the vitellogenin receptor Yolkless. In the yl 15 mutant, the receptor is still present but much accumulates internally, possibly in the ER, as seen for other yolkless alleles (Schonbaum et al., 2000).
Figure 4.
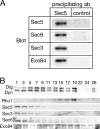
Sec5 in ovaries is assembled into the exocyst complex. (A) Protein blots with the indicated antibodies of immunoprecipitates from ovary extracts prepared from wild-type females. The precipitates were probed with sera against Sec5 or an irrelevant protein, Cog8 (Dor1), a component of the Golgi-localized COG complex (control). (B). Protein blots of fractions from a glycerol gradient separation of detergent-solubilized ovary extracts from wild-type females, probed with antisera to the indicated proteins. The four exocyst components examined peak toward the bottom of the gradient (fraction 1), with Exo84 being also present in less rapidly sedimenting fractions, which is consistent with a previous paper (Moskalenko et al., 2003). The small GTPase Rho1 (21.7 kD), Discs large (Dlg; 102.5 kD), and Shibire, the Drosophila homologue of the GTPase dynamin (Dyn; 97.8) sediment less rapidly.
Figure 5.
_sec5_E13 oocytes have a defect in trafficking of the Yolkless receptor. (A) Eggs derived from a sec5 E13 germline have a yolkless phenotype, and are often collapsed due to the lack of yolk granules. The severity of the yolkless phenotype was indistinguishable between flies raised at 18, 25, or 30°C. (B) Low magnification electron micrographs of oocytes from wild-type or sec5 E13 germlines. The vitelline bodies of the forming vitelline membrane (vb) lie between the oocyte and the follicle cells (fc). The small pale inclusions in the cytoplasm of both oocytes are lipid droplets. Bars, 5 μm. (C–E) Electron micrographs of cryosections of stage 10 oocytes labeled with an anti-Yolkless antibody followed by protein A gold (10 nm). The sec5 E13 oocyte shows reduced Yolkless labeling on and below the plasma membrane and a reduction in endocytic structures (C). There is a two- to threefold increase in labeling in the Golgi (go; yolk granule, gr) (D) and a striking appearance of Yolkless in the rim of later endocytic compartments including large uncondensed yolk granules (E). Bars, 200 nm.
Figure 6.
Yolkless accumulates in endocytic compartments in _sec5_E13 oocytes. (A) Confocal micrographs of 500-nm cryosections of egg chambers from wild-type or sec5 E13 germlines labeled with antibodies to Yolkless and a fluorescent secondary antibody. (B) Confocal micrographs of cryosections as in A, double labeled with antibodies to Yolkless and either yolk or the Cog3 subunit of the Drosophila COG complex (Golgi), which recognizes the Drosophila Golgi (Fig. S1 B). A region near the surface of the oocyte (ooc.) is shown along with adjacent follicle cells (foll.).
Figure 7.
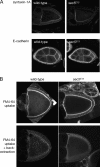
Analysis of membrane-trafficking processes in _sec5_E13 oocytes. (A) Confocal microscopic sections of egg chambers from wild-type or sec5 E13 germlines labeled with antibodies to the plasma membrane SNARE syntaxin-1A or the adhesion molecule E-cadherin. (B) Confocal micrographs of unfixed ovaries incubated with FM4-64 for 30 min. Without back extraction, the bulk of the dye is in the plasma membrane, but after back extraction at 4°C only dye taken up into the endosomal system remains. Uptake into the oocyte from the sec5 E13 germline clone is significantly reduced, whereas the surrounding, somatically derived follicle cells internalize dye at wild-type levels.
Figure 8.
Sec5 localization and function in other cell types. (A) Electron micrographs of coated pits and vesicles labeled with anti-Sec5 in cryosections of follicular epithelial cells from wild-type ovaries and S2 cultured cells. Bars, 100 nm. (B) Growth at the indicated temperatures of wild-type yeast (BY4741; wt), or the same strain with SEC5 truncated at residue 343 by insertion of a triple HA tag (_sec5-Δ_343-3xHA). (C) Confocal micrograph of live yeast in which the single copy of the SEC5 gene is truncated at residue 343 by insertion of a GFP tag. (D) Confocal micrographs of _sec5-Δ_343-3xHA strain expressing Snc1p-GFP and imaged after 4 h at 37°C. (E) Confocal micrographs of live yeast expressing a truncated form of the a-factor receptor Ste3p(Δ365) tagged in the genome with GFP. Cells were imaged after incubation for 2 h at 37°C (0 min) and then 45 min after addition of a-factor, and 30 min after a-factor was removed by washing. The truncated a-factor receptor is competent for ligand-stimulated endocytic recycling and localizes to the plasma membrane in both wild-type and _sec5-Δ_343-3xHA mutant cells. 45 min after a-factor addition, the receptor is internalized and localizes to bright dots in the cytoplasm and to the plasma membrane at the emerging schmoos in wild-type cells. In _sec5-Δ_343-3xHA cells, the receptor is internalized but shows a diffuse distribution. 30 min after a-factor was washed away, wild-type cells restore the plasma membrane localization of the receptor, whereas in the mutant cells the receptor still localizes internally.
Figure 9.
Schematic illustration of the recycling route of Yolkless. Yolkless binds yolk and both are endocytosed in clathrin-coated vesicles. Yolk condenses in early endosomes and is excluded from tubules that are thought to recycle Yolkless (dotted line). The condensing yolk (dashed line) remains in the late endosomes, which fuse together to form mature granules. Membrane fusion events that could involve Sec5 are between endocytosed vesicles (1) and between recycling tubules and the plasma membrane (2). Model based on previous microscopy and endocytosis experiments (Roth and Porter, 1964; Giorgi and Jacob, 1977; Tsuruhara et al., 1990).
Similar articles
- The exocyst component Sec5 is required for membrane traffic and polarity in the Drosophila ovary.
Murthy M, Schwarz TL. Murthy M, et al. Development. 2004 Jan;131(2):377-88. doi: 10.1242/dev.00931. Epub 2003 Dec 17. Development. 2004. PMID: 14681190 - Essential function of Drosophila Sec6 in apical exocytosis of epithelial photoreceptor cells.
Beronja S, Laprise P, Papoulas O, Pellikka M, Sisson J, Tepass U. Beronja S, et al. J Cell Biol. 2005 May 23;169(4):635-46. doi: 10.1083/jcb.200410081. Epub 2005 May 16. J Cell Biol. 2005. PMID: 15897260 Free PMC article. - Regulation of the vitellogenin receptor during Drosophila melanogaster oogenesis.
Schonbaum CP, Perrino JJ, Mahowald AP. Schonbaum CP, et al. Mol Biol Cell. 2000 Feb;11(2):511-21. doi: 10.1091/mbc.11.2.511. Mol Biol Cell. 2000. PMID: 10679010 Free PMC article. - Taking apart the endocytic machinery.
Kaksonen M. Kaksonen M. J Cell Biol. 2008 Mar 24;180(6):1059-60. doi: 10.1083/jcb.200802174. J Cell Biol. 2008. PMID: 18362177 Free PMC article. Review. - The exocyst complex in polarized exocytosis.
Hsu SC, TerBush D, Abraham M, Guo W. Hsu SC, et al. Int Rev Cytol. 2004;233:243-65. doi: 10.1016/S0074-7696(04)33006-8. Int Rev Cytol. 2004. PMID: 15037366 Review.
Cited by
- The Exocyst Complex in Health and Disease.
Martin-Urdiroz M, Deeks MJ, Horton CG, Dawe HR, Jourdain I. Martin-Urdiroz M, et al. Front Cell Dev Biol. 2016 Apr 12;4:24. doi: 10.3389/fcell.2016.00024. eCollection 2016. Front Cell Dev Biol. 2016. PMID: 27148529 Free PMC article. Review. - Local BMP receptor activation at adherens junctions in the Drosophila germline stem cell niche.
Michel M, Raabe I, Kupinski AP, Pérez-Palencia R, Bökel C. Michel M, et al. Nat Commun. 2011 Aug 2;2:415. doi: 10.1038/ncomms1426. Nat Commun. 2011. PMID: 21811244 - New roles for endosomes: from vesicular carriers to multi-purpose platforms.
Gould GW, Lippincott-Schwartz J. Gould GW, et al. Nat Rev Mol Cell Biol. 2009 Apr;10(4):287-92. doi: 10.1038/nrm2652. Epub 2009 Mar 11. Nat Rev Mol Cell Biol. 2009. PMID: 19277045 Free PMC article. Review. - The Trypanosome Exocyst: A Conserved Structure Revealing a New Role in Endocytosis.
Boehm CM, Obado S, Gadelha C, Kaupisch A, Manna PT, Gould GW, Munson M, Chait BT, Rout MP, Field MC. Boehm CM, et al. PLoS Pathog. 2017 Jan 23;13(1):e1006063. doi: 10.1371/journal.ppat.1006063. eCollection 2017 Jan. PLoS Pathog. 2017. PMID: 28114397 Free PMC article. - The synaptobrevin homologue Snc2p recruits the exocyst to secretory vesicles by binding to Sec6p.
Shen D, Yuan H, Hutagalung A, Verma A, Kümmel D, Wu X, Reinisch K, McNew JA, Novick P. Shen D, et al. J Cell Biol. 2013 Aug 5;202(3):509-26. doi: 10.1083/jcb.201211148. Epub 2013 Jul 29. J Cell Biol. 2013. PMID: 23897890 Free PMC article.
References
- Andrews, H.K., Y.Q. Zhang, N. Trotta, and K. Broadie. 2002. Drosophila sec10 is required for hormone secretion but not general exocytosis or neurotransmission. Traffic. 3:906–921. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases