The DiGEM trial protocol--a randomised controlled trial to determine the effect on glycaemic control of different strategies of blood glucose self-monitoring in people with type 2 diabetes [ISRCTN47464659] - PubMed (original) (raw)
Comparative Study
The DiGEM trial protocol--a randomised controlled trial to determine the effect on glycaemic control of different strategies of blood glucose self-monitoring in people with type 2 diabetes [ISRCTN47464659]
Andrew Farmer et al. BMC Fam Pract. 2005.
Abstract
Background: We do not yet know how to use blood glucose self-monitoring (BGSM) most effectively in the self-management of type 2 diabetes treated with oral medication. Training in monitoring may be most effective in improving glycaemic control and well being when results are linked to behavioural change.
Methods/design: DiGEM is a three arm randomised parallel group trial set in UK general practices. A total of 450 patients with type 2 diabetes managed with lifestyle or oral glucose lowering medication are included. The trial compares effectiveness of three strategies for monitoring glycaemic control over 12 months (1) a control group with three monthly HbA1c measurements; interpreted with nurse-practitioner; (2) A self-testing of blood glucose group; interpreted with nurse- practitioner to inform adjustment of medication in addition to 1; (3) A self-monitoring of blood glucose group with personal use of results to interpret results in relation to lifestyle changes in addition to 1 and 2. The trial has an 80% power at a 5% level of significance to detect a difference in change in the primary outcome, HbA1c of 0.5% between groups, allowing for an attrition rate of 10%. Secondary outcome measures include health service costs, well-being, and the intervention effect in sub-groups defined by duration of diabetes, current management, health status at baseline and co-morbidity. A mediation analysis will explore the extent to which changes in beliefs about self-management of diabetes between experimental groups leads to changes in outcomes in accordance with the Common Sense Model of illness. The study is open and has recruited more than half the target sample. The trial is expected to report in 2007.
Discussion: The DiGEM intervention and trial design address weaknesses of previous research by use of a sample size with power to detect a clinically significant change in HbA1c, recruitment from a well-characterised primary care population, definition of feasible monitoring and behaviour change strategies based on psychological theory and evidence, and measures along the hypothesised causal path from cognitions to behaviours and disease and well being related outcomes. The trial will provide evidence to support, focus or discourage use of specific BGSM strategies.
Figures
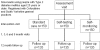
Figure 1
Consort diagram showing planned study numbers.
Similar articles
- Self-monitoring of Blood Glucose in Non-Insulin Treated Type 2 Diabetes (The SMBG Study): study protocol for a randomised controlled trial.
Parsons S, Luzio S, Bain S, Harvey J, McKenna J, Khan A, Rice S, Watkins A, Owens DR. Parsons S, et al. BMC Endocr Disord. 2017 Jan 26;17(1):4. doi: 10.1186/s12902-017-0154-x. BMC Endocr Disord. 2017. PMID: 28143495 Free PMC article. Clinical Trial. - Blood glucose self-monitoring in type 2 diabetes: a randomised controlled trial.
Farmer AJ, Wade AN, French DP, Simon J, Yudkin P, Gray A, Craven A, Goyder L, Holman RR, Mant D, Kinmonth AL, Neil HA; DiGEM Trial Group. Farmer AJ, et al. Health Technol Assess. 2009 Feb;13(15):iii-iv, ix-xi, 1-50. doi: 10.3310/hta13150. Health Technol Assess. 2009. PMID: 19254484 Clinical Trial. - Effect of 6 months' flash glucose monitoring in adolescents and young adults with type 1 diabetes and suboptimal glycaemic control: managing diabetes in a 'flash' randomised controlled trial protocol.
Boucher SE, Gray AR, de Bock M, Wiltshire EJ, Galland BC, Tomlinson PA, Rayns J, MacKenzie KE, Wheeler BJ. Boucher SE, et al. BMC Endocr Disord. 2019 May 20;19(1):50. doi: 10.1186/s12902-019-0378-z. BMC Endocr Disord. 2019. PMID: 31109342 Free PMC article. Clinical Trial. - Self-monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin.
Malanda UL, Welschen LM, Riphagen II, Dekker JM, Nijpels G, Bot SD. Malanda UL, et al. Cochrane Database Syst Rev. 2012 Jan 18;1:CD005060. doi: 10.1002/14651858.CD005060.pub3. Cochrane Database Syst Rev. 2012. PMID: 22258959 Review. - Repaglinide : a pharmacoeconomic review of its use in type 2 diabetes mellitus.
Plosker GL, Figgitt DP. Plosker GL, et al. Pharmacoeconomics. 2004;22(6):389-411. doi: 10.2165/00019053-200422060-00005. Pharmacoeconomics. 2004. PMID: 15099124 Review.
Cited by
- Randomized studies are needed to assess the true role of self-monitoring of blood glucose in noninsulin-treated type 2 diabetes.
Parkin CG, Price D. Parkin CG, et al. J Diabetes Sci Technol. 2007 Jul;1(4):595-602. doi: 10.1177/193229680700100419. J Diabetes Sci Technol. 2007. PMID: 19885124 Free PMC article. - Protocol for the ADDITION-Plus study: a randomised controlled trial of an individually-tailored behaviour change intervention among people with recently diagnosed type 2 diabetes under intensive UK general practice care.
Griffin SJ, Simmons RK, Williams KM, Prevost AT, Hardeman W, Grant J, Whittle F, Boase S, Hobbis I, Brage S, Westgate K, Fanshawe T, Sutton S, Wareham NJ, Kinmonth AL; ADDITION-Plus study team. Griffin SJ, et al. BMC Public Health. 2011 Apr 4;11:211. doi: 10.1186/1471-2458-11-211. BMC Public Health. 2011. PMID: 21463520 Free PMC article. Clinical Trial. - Comment on: Davidson MB (2007) The dilemma of self-monitoring of blood glucose. Diabetologia 50:497-499.
Kolb H, Martin S, Karter AJ. Kolb H, et al. Diabetologia. 2007 Jul;50(7):1565-6; author reply 1567-8. doi: 10.1007/s00125-007-0693-1. Epub 2007 May 8. Diabetologia. 2007. PMID: 17486313 Free PMC article. No abstract available. - Cost effectiveness of self monitoring of blood glucose in patients with non-insulin treated type 2 diabetes: economic evaluation of data from the DiGEM trial.
Simon J, Gray A, Clarke P, Wade A, Neil A, Farmer A; Diabetes Glycaemic Education and Monitoring Trial Group. Simon J, et al. BMJ. 2008 May 24;336(7654):1177-80. doi: 10.1136/bmj.39526.674873.BE. Epub 2008 Apr 17. BMJ. 2008. PMID: 18420663 Free PMC article. Clinical Trial. - Challenges of maintaining research protocol fidelity in a clinical care setting: a qualitative study of the experiences and views of patients and staff participating in a randomized controlled trial.
Lawton J, Jenkins N, Darbyshire JL, Holman RR, Farmer AJ, Hallowell N. Lawton J, et al. Trials. 2011 May 4;12:108. doi: 10.1186/1745-6215-12-108. Trials. 2011. PMID: 21542916 Free PMC article. Clinical Trial.
References
- Wild S, Roglic G, Green A, Sicree R, King H. Global Prevalence of Diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. - PubMed
- Garfield SA, Malozowski S, Chin MH, Venkat Narayan KM, Glasgow RE, Green LW, Hiss RG, Krumholz HM. Considerations for Diabetes Translational Research in Real-World Settings. Diabetes Care. 2003;26:2670–2674. - PubMed
- Norris SL, Lau J, Smith SJ, Schmid CH, Engelgau MM. Self-Management Education for Adults With Type 2 Diabetes: A meta-analysis of the effect on glycemic control. Diabetes Care. 2002;25:1159–1171. - PubMed
- Blonde L, Ginsberg BH, Horn S. Frequency of blood glucose monitoring in relation to glycaemic control in patients with type 2 diabetes. Diabetes Care. 2002;25:245–246. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical