P2X2 knockout mice and P2X2/P2X3 double knockout mice reveal a role for the P2X2 receptor subunit in mediating multiple sensory effects of ATP - PubMed (original) (raw)
. 2005 Sep 1;567(Pt 2):621-39.
doi: 10.1113/jphysiol.2005.088435. Epub 2005 Jun 16.
Philip M Dunn, Yu Zhong, Weifang Rong, Sara G Hamilton, Gillian E Knight, Huai-Zhen Ruan, Bei Ma, Ping Yip, Philip Nunn, Stephen B McMahon, Geoffrey Burnstock, Anthony P D W Ford
Affiliations
- PMID: 15961431
- PMCID: PMC1474198
- DOI: 10.1113/jphysiol.2005.088435
P2X2 knockout mice and P2X2/P2X3 double knockout mice reveal a role for the P2X2 receptor subunit in mediating multiple sensory effects of ATP
Debra A Cockayne et al. J Physiol. 2005.
Abstract
Extracellular ATP plays a role in nociceptive signalling and sensory regulation of visceral function through ionotropic receptors variably composed of P2X2 and P2X3 subunits. P2X2 and P2X3 subunits can form homomultimeric P2X2, homomultimeric P2X3, or heteromultimeric P2X2/3 receptors. However, the relative contribution of these receptor subtypes to afferent functions of ATP in vivo is poorly understood. Here we describe null mutant mice lacking the P2X2 receptor subunit (P2X2-/-) and double mutant mice lacking both P2X2 and P2X3 subunits (P2X2/P2X3(Dbl-/-)), and compare these with previously characterized P2X3-/- mice. In patch-clamp studies, nodose, coeliac and superior cervical ganglia (SCG) neurones from wild-type mice responded to ATP with sustained inward currents, while dorsal root ganglia (DRG) neurones gave predominantly transient currents. Sensory neurones from P2X2-/- mice responded to ATP with only transient inward currents, while sympathetic neurones had barely detectable responses. Neurones from P2X2/P2X3(Dbl-/-) mice had minimal to no response to ATP. These data indicate that P2X receptors on sensory and sympathetic ganglion neurones involve almost exclusively P2X2 and P2X3 subunits. P2X2-/- and P2X2/P2X3(Dbl-/-) mice had reduced pain-related behaviours in response to intraplantar injection of formalin. Significantly, P2X3-/-, P2X2-/-, and P2X2/P2X3(Dbl-/-) mice had reduced urinary bladder reflexes and decreased pelvic afferent nerve activity in response to bladder distension. No deficits in a wide variety of CNS behavioural tests were observed in P2X2-/- mice. Taken together, these data extend our findings for P2X3-/- mice, and reveal an important contribution of heteromeric P2X2/3 receptors to nociceptive responses and mechanosensory transduction within the urinary bladder.
Figures
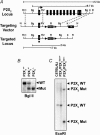
Figure 1. Targeted disruption of the P2X2 gene and generation of P2X2−/− and P2X2/P2X3Dbl−/− mice
A, gene targeting strategy showing the 12 kb genomic fragment of the mouse P2X2 gene used for deletion of exons (filled bars) 2–11. Dashed lines represent the 5′ and 3′ genomic arms of the targeting vector. Position of the _LoxP_-flanked Neo and TK selection markers are indicated. Restriction sites indicated are R, _Eco_RI; H, _Hin_dIII; Bg, _Bgl_II; RV, _Eco_RV; K, _Kpn_I; N, _Not_I. A, _Not_I linearized targeting vector was transfected in 129Ola E14-1 ES cells to generate the targeted P2X2 locus. B, _Bgl_II Southern blot used for routine genotyping of P2X2+/+, P2X2+/− and P2X2−/− mice using the indicated exon-1-specific probe. P2X2/P2X3 double knockout mice were generated by conventional backcrossing as described in Methods. C, _Eco_R1 Southern blot of C57BL/6 J, P2X2/P2X3Dbl+/+ and P2X2/P2X3Dbl−/− mice probed simultaneously with a P2X2 exon-1-specific probe and a P2X3 5′ flanking region probe (Cockayne et al. 2000). The P2X2 and P2X3 wild-type alleles in the P2X2/P2X3Dbl+/+ mice are represented by the expected 12 and 3 kb fragments, respectively, and the P2X2 and P2X3 mutant alleles in P2X2/P2X3Dbl−/− mice are represented by the expected 7 and 1.5 kb fragments, respectively.
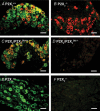
Figure 2. Double immunofluorescence labelling of P2X2 and P2X3 receptors in sensory and sympathetic neurones
A–D, immunofluorescence doubling labelling for P2X2 (green) and P2X3 (red) receptors in transverse sections (10 μm) of DRG (colocalization, yellow) neurones from P2X2+/+ (A), P2X2−/− (B), P2X2/P2X3Dbl+/+ (C) and P2X2/P2X3Dbl−/− (D) mice. P2X2 and P2X3 immunoreactivity was present in small-large and small-medium cells, respectively, in P2X2+/+ (A) and P2X2/P2X3Dbl+/+ (C) mice, and many DRG neurones showed colocalization of P2X2 and P2X3 receptors. P2X2 immunoreactivity was absent in P2X2−/− mice (B), while P2X3 immunoreactivity appeared unaltered. Both P2X2 and P2X3 immunoreactivity was absent in P2X2/P2X3Dbl−/− mice (D). E and F, immunofluorescence doubling labelling for P2X2 (green) and P2X3 (red) receptors in transverse sections (10 μm) of superior cervical ganglia (SCG) neurones from P2X2+/+ (E) and P2X2−/− (F) mice. Many SCG neurones in P2X2+/+ mice showed P2X2 receptor expression (E), and this P2X2 immunoreactivity was absent in P2X2−/− mice (F). Specific P2X3 immunoreactivity was not observed in SCG neurones of either P2X2+/+ or P2X2−/− mice. Scale bars, 50 μ
m
.
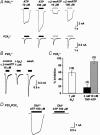
Figure 3. Whole-cell patch-clamp recordings of DRG neurones from P2X2−/−, P2X3−/− and P2X2/P2X3Dbl−/− mice in response to P2X agonists
A, wild-type dorsal root ganglia (DRG) neurones (representative traces from P2X2/P2X3Dbl+/+ mice) responded to ATP and α,β-meATP with either rapidly desensitizing (a) or sustained (b) responses; a composite response having both rapidly and slowly desensitizing components was also observed in some neurones (data not shown). All DRG neurones examined responded to 100 μ
m
GABA with a sustained inward current. Comparable responses were seen in DRG neurones from all wild-type lines. B, in P2X2−/− mice, DRG neurones all responded to ATP and α,β-meATP with rapidly desensitizing transient responses. C, in P2X3−/− mice, many DRG neurones failed to respond to either ATP or α,β-meATP, but did respond to 100 μ
m
GABA (a). Other P2X3−/− neurones responded to ATP with a sustained inward current, but failed to respond to α,β-meATP (b). D, in P2X2/P2X3Dbl−/− mice, most DRG neurones failed to respond to ATP or α,β-meATP, but did respond to 100 μ
m
GABA (a). A small percentage of neurones in double knockout mice gave small, very low amplitude responses to ATP (b), but did not respond to α,β-meATP (data not shown).
Figure 4. Whole-cell patch-clamp recordings of nodose ganglion neurones from P2X2−/− and P2X2/P2X3Dbl−/− mice in response to P2X agonists
A, comparison of responses to ATP and α,β-meATP recorded from nodose ganglion neurones of P2X2+/+ and P2X2−/− mice. Wild-type neurones gave a sustained inward current to both agonists, while neurones from P2X2−/− mice responded with a rapidly desensitizing transient response. B and C, antagonist sensitivity of the remaining α,β-meATP response recorded in P2X2−/− nodose neurones. B, representative responses to 10 μ
m
α,β-meATP recorded from a single nodose neurone before, in the presence of, and after washing out the antagonist 1 μ
m
diinosine pentaphosphate (Ip5I). C, histogram comparing the antagonist effect of 1 μ
m
Ip5I with that of 3 n
m
and 10 n
m
TNP-ATP. Data represent means ±
s.e.m.
from the number of neurones shown in parenthesis. D, comparison of responses to ATP recorded in nodose ganglion neurones from P2X2/P2X3Dbl+/+ and P2X2/P2X3Dbl−/− mice.
Figure 5. Whole-cell patch-clamp recordings of SCG neurones from P2X2−/− mice in response to ATP
A, SCG neurones from P2X2+/+ mice gave a robust sustained inward current to ATP and the nicotinic agonist DMPP, but not to α,β-meATP. B, SCG neurones from P2X2−/− mice responded to DMPP, but there was no response to ATP or α,β-meATP.
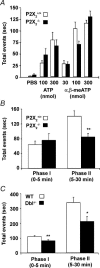
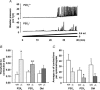
Figure 6. P2X agonist and formalin-evoked nociceptive responses in P2X2−/− and P2X2/P2X3Dbl−/− mice
A, hindpaw lifting response in 2- to 3-month-old female P2X2+/+ (open bars) and P2X2−/− (filled bars) mice (n = 8–10) following intraplantar injection of varying doses of ATP or α,β-meATP in a total volume of 30 μl. Data represent the means ±
s.e.m.
of the total time spent lifting the treated paw in a 4 min time bin following administration of compound. B and C, nociceptive behavioural response in 3-month-old male and female P2X2+/+ (B, open bars) and P2X2−/− (B, filled bars) mice (n = 9–11), or 3- to 4-month-old male P2X2/P2X3Dbl+/+ (C, open bars) and P2X2/P2X3Dbl−/− (C, filled bars) mice (n = 12) following intraplantar injection of 5% formalin in a total volume of 20 μl. Data represent the means ±
s.e.m.
of the total time spent licking, biting or flinching the treated paw in the 0–5 and 5–30 min time bins following administration of formalin. *P < 0.05, **P < 0.01; Wilcoxon rank-sum exact test.
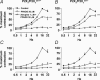
Figure 7. Urinary bladder cystometry in P2X3−/−, P2X2−/− and P2X2/P2X3Dbl−/− mice
A, representative acute cystometrograms recorded from anaesthetized and transurethrally catheterized 5- to 6-month-old female P2X2+/+ and P2X2−/− mice. Traces illustrate bladder pressure recorded in response to a constant intravesical infusion of saline (10 μl min−1 for 40 min). Contractions greater than 20 cm H2O were taken as micturition contractions. B and C, quantification of the threshold for contraction (B) and the average number of contractions per cystometrogram (C) for 5- to 6-month-old female P2X3−/− (n = 7), P2X2−/− (n = 10) and P2X2/P2X3Dbl−/− (n = 7) mice tested in parallel, under similar conditions, with their respective wild-type controls (P2X3+/+ and P2X2/P2X3Dbl+/+n = 7, P2X2+/+n = 11). Data represent the means ±
s.e.m.
*P < 0.05, **P < 0.01; Wilcoxon rank-sum exact test.
Figure 8. Frequency–response curves of the purinergic and cholinergic components of nerve-mediated responses in bladders of P2X2/P2X3Dbl−/− mice
Representative frequency–response curves in the absence (control) and presence of PPADS (30 μ
m
), and finally in the presence of both PPADS (30 μ
m
) and atropine (1 μ
m
) for (A) male P2X2/P2X3Dbl+/+ mice (n = 5), (B) male P2X2/P2X3Dbl−/− mice (n = 6), (C) female P2X2/P2X3Dbl+/+ mice (n = 5), and (D) female P2X2/P2X3Dbl−/− mice (n = 5). Electrical field stimulation (EFS) was carried out at 100 V, 0.3 ms, 0.5–32 Hz, with 15 s stimulation. Data represent the means ±
s.e.m.
of the percentage of the maximum control response. ***P < 0.001; two-way analysis of variance (ANOVA).
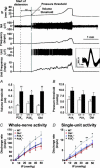
Figure 9. Pelvic nerve response to bladder filling in P2X3−/−, P2X2−/− and P2X2/P2X3Dbl−/− mice
Representative traces of intraluminal pressure (IP), whole-nerve activity (NA), and whole-nerve discharge rate (frequency) are shown for P2X3 (left), P2X2 (middle) and P2X2/P2X3Dbl (right) wild-type (upper panels) and mutant (lower panels) mice. The dotted lines indicate the start of bladder distension at a constant rate of 0.1 ml min−1. Note the marked delay in bladder afferent activation in P2X-deficient mice.
Figure 10. Threshold for whole-nerve response to bladder distension and single unit discrimination reveals a reduced sensitivity of pelvic afferents in P2X-deficient mice
Top panel, the upper three traces are intraluminal pressure (IP), whole-nerve activity (NA) and whole-nerve discharge rate (frequency). The lower two traces are a single-unit spike (Unit) and its discharge rate (unit frequency). In the inset are superimposed spikes of that unit showing little variation in waveform. The dotted line indicates the start of bladder filling and the second solid line indicates the start of nerve firing (pressure threshold). Volume threshold is calculated from the interval between those two lines and the rate of bladder filling (0.1 ml min−1). Bottom panel, reduced sensitivity of pelvic afferents to bladder filling in P2X3−/−, P2X2−/− and P2X2/P2X3Dbl−/− mice. A, volume thresholds were significantly increased in P2X-deficient mice (n = 7–10) compared with the respective wild-type controls (n = 6–12). B, pressure thresholds were not significantly different between P2X-deficient mice (n = 6–10) and wild-type controls (n = 6–9). C, the pressure–response curves of whole nerves (C) and single units (D) in P2X-deficient mice showed shallower slopes compared with wild-type mice. *P < 0.05 and **P < 0.01 comparing the mean discharge frequency of wild-type mice with P2X-deficient mice at various intraluminal pressure levels; two way analysis of variance (ANOVA).
Similar articles
- Changes in P2X receptor responses of sensory neurons from P2X3-deficient mice.
Zhong Y, Dunn PM, Bardini M, Ford AP, Cockayne DA, Burnstock G. Zhong Y, et al. Eur J Neurosci. 2001 Dec;14(11):1784-92. doi: 10.1046/j.0953-816x.2001.01805.x. Eur J Neurosci. 2001. PMID: 11860473 - Mechanical allodynia caused by intraplantar injection of P2X receptor agonist in rats: involvement of heteromeric P2X2/3 receptor signaling in capsaicin-insensitive primary afferent neurons.
Tsuda M, Koizumi S, Kita A, Shigemoto Y, Ueno S, Inoue K. Tsuda M, et al. J Neurosci. 2000 Aug 1;20(15):RC90. doi: 10.1523/JNEUROSCI.20-15-j0007.2000. J Neurosci. 2000. PMID: 10899177 Free PMC article. - Double P2X2/P2X3 purinergic receptor knockout mice do not taste NaCl or the artificial sweetener SC45647.
Eddy MC, Eschle BK, Barrows J, Hallock RM, Finger TE, Delay ER. Eddy MC, et al. Chem Senses. 2009 Nov;34(9):789-97. doi: 10.1093/chemse/bjp068. Chem Senses. 2009. PMID: 19833661 Free PMC article. - [ATP receptors in pain].
Tsuda M, Koizumi S, Inoue K. Tsuda M, et al. Nihon Yakurigaku Zasshi. 2000 Dec;116(6):343-50. doi: 10.1254/fpj.116.343. Nihon Yakurigaku Zasshi. 2000. PMID: 11188502 Review. Japanese. - Homomeric and heteromeric P2X3 receptors in peripheral sensory neurons.
Brederson JD, Jarvis MF. Brederson JD, et al. Curr Opin Investig Drugs. 2008 Jul;9(7):716-25. Curr Opin Investig Drugs. 2008. PMID: 18600577 Review.
Cited by
- Botulinum neurotoxin serotype A suppresses neurotransmitter release from afferent as well as efferent nerves in the urinary bladder.
Ikeda Y, Zabbarova IV, Birder LA, de Groat WC, McCarthy CJ, Hanna-Mitchell AT, Kanai AJ. Ikeda Y, et al. Eur Urol. 2012 Dec;62(6):1157-64. doi: 10.1016/j.eururo.2012.03.031. Epub 2012 Mar 23. Eur Urol. 2012. PMID: 22480459 Free PMC article. - Purinergic P2X7 receptors as therapeutic targets in interstitial cystitis/bladder pain syndrome; key role of ATP signaling in inflammation.
Taidi Z, Mansfield KJ, Bates L, Sana-Ur-Rehman H, Liu L. Taidi Z, et al. Bladder (San Franc). 2019 Apr 8;6(1):e38. doi: 10.14440/bladder.2019.789. eCollection 2019. Bladder (San Franc). 2019. PMID: 32775480 Free PMC article. Review. - Functional profiling of neurons through cellular neuropharmacology.
Teichert RW, Smith NJ, Raghuraman S, Yoshikami D, Light AR, Olivera BM. Teichert RW, et al. Proc Natl Acad Sci U S A. 2012 Jan 31;109(5):1388-95. doi: 10.1073/pnas.1118833109. Epub 2012 Jan 23. Proc Natl Acad Sci U S A. 2012. PMID: 22307590 Free PMC article. - Purinoceptors as therapeutic targets for lower urinary tract dysfunction.
Ford AP, Gever JR, Nunn PA, Zhong Y, Cefalu JS, Dillon MP, Cockayne DA. Ford AP, et al. Br J Pharmacol. 2006 Feb;147 Suppl 2(Suppl 2):S132-43. doi: 10.1038/sj.bjp.0706637. Br J Pharmacol. 2006. PMID: 16465177 Free PMC article. Review. - Cyclophosphamide-induced bladder inflammation sensitizes and enhances P2X receptor function in rat bladder sensory neurons.
Dang K, Lamb K, Cohen M, Bielefeldt K, Gebhart GF. Dang K, et al. J Neurophysiol. 2008 Jan;99(1):49-59. doi: 10.1152/jn.00211.2007. Epub 2007 Oct 24. J Neurophysiol. 2008. PMID: 17959738 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases