Modeling dynamic reciprocity: engineering three-dimensional culture models of breast architecture, function, and neoplastic transformation - PubMed (original) (raw)
Review
Modeling dynamic reciprocity: engineering three-dimensional culture models of breast architecture, function, and neoplastic transformation
Celeste M Nelson et al. Semin Cancer Biol. 2005 Oct.
Abstract
In order to understand why cancer develops as well as predict the outcome of pharmacological treatments, we need to model the structure and function of organs in culture so that our experimental manipulations occur under physiological contexts. This review traces the history of the development of a prototypic example, the three-dimensional (3D) model of the mammary gland acinus. We briefly describe the considerable information available on both normal mammary gland function and breast cancer generated by the current model and present future challenges that will require an increase in its complexity. We propose the need for engineered tissues that faithfully recapitulate their native structures to allow a greater understanding of tissue function, dysfunction, and potential therapeutic intervention.
Figures
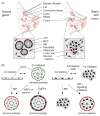
Fig. 1
Structure and 3D model of mammary gland. (A) Luminal epithelial cells (LEP) forming the acinus are regulated by homotypic interactions with neighboring luminal cells, by heterotypic interactions with myoepithelial cells (MEP), and by interactions with the BM. The bi-layered structure is surrounded by a complex BM and a stroma comprised of stromal ECM, fibroblasts, and adipocytes, as well as nerves, blood, and lymphatic vessels (not depicted). This structure is disrupted in tumors. Additionally, myoepithelial cells surrounding the tumor have altered function [105,130]. (B) (left) In the 3D model of the normal mammary gland acinus, human or mouse mammary epithelial cells cultured in or on laminin-1-rich, malleable BM will form spherical structures with a central lumen. Addition of lactogenic hormones to mouse cells leads to the synthesis and vectorial secretion of milk proteins into the lumen (milk production by non-malignant human epithelial cells has not yet been achieved). Luminal epithelial cells fail to organize into an acinus when cultured in 3D collagen gels unless they receive signals from myoepithelial cells, which synthesize the laminin components of BM. (right) Oncogenic insults prevent tumorigenic epithelial cells from forming the acinus; these tumorigenic structures can be functionally reverted by restoring normal signaling pathways [52]. (B) modified from [131]. Red, BM; blue, MEPs; white/grey, LEPs; green, sialomucin.
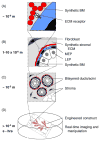
Fig. 2
The tissue-engineered breast. New strategies should enable the control of the microenvironment at the nano-, micro-, and macro-scales with temporal precision. (A) Synthetic and recombinant ECM polymers impart cues sensed directly by cell-surface receptors. (B–C) Microfabricated constructs control the positions of multiple cell types with respect to each other and the ECM with micrometer precision across large areas of tissue. (D) Engineered breast tissues that can be visualized and manipulated in real time.
Similar articles
- Engineering mammary gland in vitro models for cancer diagnostics and therapy.
Campbell JJ, Hume RD, Watson CJ. Campbell JJ, et al. Mol Pharm. 2014 Jul 7;11(7):1971-81. doi: 10.1021/mp500121c. Epub 2014 Apr 25. Mol Pharm. 2014. PMID: 24766393 Review. - Organoid models for mammary gland dynamics and breast cancer.
Srivastava V, Huycke TR, Phong KT, Gartner ZJ. Srivastava V, et al. Curr Opin Cell Biol. 2020 Oct;66:51-58. doi: 10.1016/j.ceb.2020.05.003. Epub 2020 Jun 11. Curr Opin Cell Biol. 2020. PMID: 32535255 Free PMC article. Review. - Three-dimensional cell culture to model epithelia in the female reproductive system.
Adissu HA, Asem EK, Lelièvre SA. Adissu HA, et al. Reprod Sci. 2007 Dec;14(8 Suppl):11-9. doi: 10.1177/1933719107310872. Reprod Sci. 2007. PMID: 18089605 Review. - The Emerging Picture of Human Breast Cancer as a Stem Cell-based Disease.
Cobaleda C, Cruz JJ, González-Sarmiento R, Sánchez-García I, Pérez-Losada J. Cobaleda C, et al. Stem Cell Rev. 2008 Summer;4(2):67-79. doi: 10.1007/s12015-008-9012-6. Stem Cell Rev. 2008. PMID: 18401767 Review. - Evaluating chemical effects on mammary gland development: A critical need in disease prevention.
Osborne G, Rudel R, Schwarzman M. Osborne G, et al. Reprod Toxicol. 2015 Jul;54:148-55. doi: 10.1016/j.reprotox.2014.07.077. Epub 2014 Aug 1. Reprod Toxicol. 2015. PMID: 25091782
Cited by
- Enzymatic Noncovalent Synthesis.
He H, Tan W, Guo J, Yi M, Shy AN, Xu B. He H, et al. Chem Rev. 2020 Sep 23;120(18):9994-10078. doi: 10.1021/acs.chemrev.0c00306. Epub 2020 Aug 19. Chem Rev. 2020. PMID: 32812754 Free PMC article. Review. - Biphenotypic Differentiation of Pancreatic Cancer in 3-Dimensional Culture.
Matsushita Y, Smith B, Delannoy M, Trujillo MA, Chianchiano P, McMillan R, Kamiyama H, Liang H, Thompson ED, Hruban RH, Matsui W, Wood LD, Roberts NJ, Eshleman JR. Matsushita Y, et al. Pancreas. 2019 Oct;48(9):1225-1231. doi: 10.1097/MPA.0000000000001390. Pancreas. 2019. PMID: 31593010 Free PMC article. - Unraveling the microenvironmental influences on the normal mammary gland and breast cancer.
Weigelt B, Bissell MJ. Weigelt B, et al. Semin Cancer Biol. 2008 Oct;18(5):311-21. doi: 10.1016/j.semcancer.2008.03.013. Epub 2008 Mar 26. Semin Cancer Biol. 2008. PMID: 18455428 Free PMC article. Review. - Breast cancer by proxy: can the microenvironment be both the cause and consequence?
Rønnov-Jessen L, Bissell MJ. Rønnov-Jessen L, et al. Trends Mol Med. 2009 Jan;15(1):5-13. doi: 10.1016/j.molmed.2008.11.001. Epub 2008 Dec 16. Trends Mol Med. 2009. PMID: 19091631 Free PMC article. - YB-1 drives preneoplastic progression: Insight into opportunities for cancer prevention.
Davies AH, Dunn SE. Davies AH, et al. Oncotarget. 2011 May;2(5):401-6. doi: 10.18632/oncotarget.276. Oncotarget. 2011. PMID: 21576761 Free PMC article.
References
- Mettlin C. Global breast cancer mortality statistics. CA Cancer J Clin. 1999;49:138–44. - PubMed
- Hortobagyi GN. Opportunities and challenges in the development of targeted therapies. Semin Oncol. 2004;31:21–7. - PubMed
- Abbott A. Cell culture: biology’s new dimension. Nature. 2003;424:870–2. - PubMed
- Osborne MP. Breast development and anatomy. Philadelphia: Lippincott-Raven Publishers; 1996.
- Russo J, Russo IH. Development pattern of human breast and susceptibility to carcinogenesis. Eur J Cancer Prev. 1993;2(Suppl 3):85–100. - PubMed
Publication types
MeSH terms
Grants and funding
- R37 CA064786/CA/NCI NIH HHS/United States
- R01 CA064786-05/CA/NCI NIH HHS/United States
- R01 CA057621/CA/NCI NIH HHS/United States
- CA64786/CA/NCI NIH HHS/United States
- CA57621/CA/NCI NIH HHS/United States
- R01 CA057621-07/CA/NCI NIH HHS/United States
- R01 CA064786/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous