A TrkA-to-p75NTR molecular switch activates amyloid beta-peptide generation during aging - PubMed (original) (raw)
A TrkA-to-p75NTR molecular switch activates amyloid beta-peptide generation during aging
Claudio Costantini et al. Biochem J. 2005.
Abstract
Aging is the single most important risk factor for AD (Alzheimer's disease). However, the molecular events that connect normal aging to AD are mostly unknown. The abnormal accumulation of Abeta (amyloid beta-peptide) in the form of senile plaques is one of the main characteristics of AD. In the present study, we show that two members of the neurotrophin receptor superfamily, TrkA (tyrosine kinase receptor A) and p75NTR (p75 neurotrophin receptor), differentially regulate the processing of APP (amyloid precursor protein): TrkA reduces, whereas p75NTR activates, beta-cleavage of APP. The p75NTR-dependent effect requires NGF (nerve growth factor) binding and activation of the second messenger ceramide. We also show that normal aging activates Abeta generation in the brain by 'switching' from the TrkA to the p75NTR receptor system. Such an effect is abolished in p75NTR 'knockout' animals, and can be blocked by both caloric restriction and inhibitors of nSMase (neutral sphingomyelinase). In contrast with caloric restriction, which prevents the age-associated up-regulation of p75NTR expression, nSMase inhibitors block the activation of ceramide. When taken together, these results indicate that the p75NTR-ceramide signalling pathway activates the rate of Abeta generation in an age-dependent fashion, and provide a new target for both the understanding and the prevention of late-onset AD.
Figures
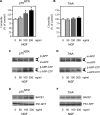
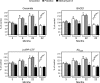
Figure 1. p75NTR and TrkA differentially regulate APP processing
p75NTR and TrkA stably transfected SK-N-BE cells were treated with increasing concentrations of NGF for 48 h. (A and B) Endogenous ceramide was analysed as described in the Materials and methods section. *Significant difference from 0 ng/ml (no treatment). Error bars represent the S.D. for three different determinations. (C and D) Western blot analysis of APP-CTFs following NGF treatment. Total cell lysates were separated on 4–12% Bis-Tris SDS/PAGE gels, blotted on to a PVDF membrane, and probed with antibody AB5352 against the C-terminal domain of APP. The primary antibody was followed by a horseradish-peroxidase-conjugated monoclonal antibody and detected by chemiluminescence as described previously [11,21]. Mature (m-APP) and immature (im-APP) APP, together with β- and α-APP-CTFs, are indicated. (E and F) Western blot analysis of BACE1 and PS1-N-terminal-fragment (PS1-NTF) expression levels following NGF treatment. Total cell lysates were separated on 4–12% Bis-Tris SDS/PAGE gels, blotted on to a PVDF membrane, and probed with either antibody ab2077 (anti-BACE) or sc-8040 (anti-PS1). The primary antibody was followed by an horseradish-peroxidase-conjugated monoclonal antibody and was detected by chemiluminescence. BACE1 and PS1-NTF are indicated.
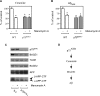
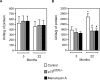
Figure 2. Ceramide is required for the p75NTR-mediated regulation of Aβ generation
(A–C) Primary neurons from WT and p75 _NTR_−/− animals were prepared as described in the Materials and methods section, and were then incubated in the absence or presence of the nSMase inhibitor manumycin A (100 μM). (A) Endogenous ceramide was analysed by TLC following prelabelling with [9,10-3H(N)]palmitic acid, as described in the Materials and methods section. (B) Aβ levels in the conditioned media were determined by standard sandwich ELISA using an anti-rodent Aβ antibody, as described in the Materials and methods section. The average concentration of Aβtotal found in the conditioned media of WT neurons was 11984±995 pmol/mg of protein. No difference in the Aβ42/Aβtotal ratio was observed when comparing WT and p75 _NTR_−/− neurons (results not shown). *Significant difference from WT neurons (no treatment). Error bars represent the S.D. for six different determinations. (C) Western blot analysis of WT and p75 _NTR_−/− neurons maintained in the absence or presence of manumycin A. Total cell lysates were separated on 4–12% Bis-Tris SDS/PAGE gels, blotted on to a PVDF membrane, and probed with the appropriate antibodies (indicated in the Materials and methods section). (D) Schematic view to summarize the results observed with SK-N-BE (Figure 1) and primary neurons (Figure 2) showing that p75NTR acts upstream of ceramide in the regulation of BACE1 steady-state levels and Aβ generation.
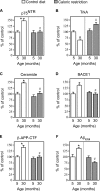
Figure 3. Caloric restriction blocks the age-associated activation of Aβ generation by acting upstream of TrkA/p75NTR
Analysis of the cerebral cortex from WT mice fed either a control (84 kcal/week) or caloric-restricted (63 kcal/week) diet. The expression levels of p75NTR (A), TrkA (B), BACE1 (D) and β-APP-CTF (E) were analysed by Western blot with the appropriate antibodies (as indicated in the Materials and methods section). Relative densities of images were calculated with the EpiChemi3 Darkroom™ (UVP Bioimaging Systems) using Labworks Image Acquisition and Analysis Software 4.5. Results are expressed as a percentage of the data for 5-month-old controls. (C) Ceramide was quantified by both ESI–MS and TLC as described in the Materials and methods section. (F) Aβ levels were determined by standard sandwich ELISA using an anti-rodent Aβ antibody, as described in the Materials and methods section. The average values of endogenous Aβtotal obtained for 5-month-old animals were 7.8±1.1 pmol/mg. Aβ42 was constantly found to be approx. 25–30% of total Aβ values. Error bars represent the S.D. for nine different determinations. Statistical significance compared with 5-month-old (*) or age-matched (#) control animals is indicated.
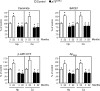
Figure 4. Genetic disruption of p75NTR abolishes the age-associated activation of Aβ generation
Analysis of both hippocampus (Hp) and cortex (Cx) from young and old p75 _NTR_−/− mice. Analysis was performed as described in the legend to Figure 3. Results are expressed as percentage of the data for 3-month-old control animals. Error bars represent the S.D. for 12 different determinations. Statistical significance compared with 3-month-old (*) or age-matched (#) control animals is indicated.
Figure 5. Manumycin A blocks the effects produced by normal aging
Mice of 1, 7, and 20 months of age were implanted with slow-release pellets containing either manumycin A (3.5 mg/animal over a period of 2 months) or placebo. Animals were killed following 2 months of treatment (at 3, 9 and 22 months of age), and were analysed as described in the legend to Figure 3. Results are expressed as a percentage of the data for 3-month-old control animals. Error bars represent the S.D. for 12 different determinations. Statistical significance compared with 3-month-old (*) or age-matched (#) control animals is indicated.
Figure 6. Enzymatic activity of β-secretase increases during aging and is under the control of the p75NTR–ceramide signalling pathway
Brain homogenates from mice cortex were assayed in vitro for both γ- (A) and β- (B) secretase activity. Each determination was done in duplicate and at two different concentrations (50 μg and 100 μg). Values are expressed as arbitrary units/g of protein, and are calculated over background levels (blank). Boiled samples were always below background levels (results not shown). Error bars represent the S.D. of four different assays. Statistical significance compared with 3-month-old (*) or age-matched (#) control animals is indicated.
Figure 7. Schematic diagram summarizing the results of the present study showing the age-associated regulation of Aβ generation
Aging regulates the rate of Aβ generation by controlling the relative expression of neurotrophin receptors p75NTR and TrkA. Upon binding to NGF, p75NTR activates nSMase and induces hydrolysis of sphingomyelin with consequent liberation of the second messenger ceramide. The ceramide-dependent signalling cascade increases the steady-state levels of BACE1, the rate-limiting enzyme in the biosynthesis of Aβ. Aβ then aggregates and precipitates in the brain in the form of senile (or amyloid) plaques. Even though the ceramide-dependent signalling cascade can also activates α-cleavage of APP (the present study and [11]), such an event does not lead to the generation of Aβ. Caloric restriction prevents the age-associated switch from the TrkA- to p75NTR-dependent signalling cascade. It is noteworthy that TrkA reduces APP processing and prevents Aβ generation. In contrast with caloric restriction, nSMase inhibitors block the generation of ceramide and prevent Aβ biosynthesis without affecting the expression of neurotrophin receptors.
Similar articles
- Dissecting the involvement of tropomyosin-related kinase A and p75 neurotrophin receptor signaling in NGF deficit-induced neurodegeneration.
Capsoni S, Tiveron C, Vignone D, Amato G, Cattaneo A. Capsoni S, et al. Proc Natl Acad Sci U S A. 2010 Jul 6;107(27):12299-304. doi: 10.1073/pnas.1007181107. Epub 2010 Jun 21. Proc Natl Acad Sci U S A. 2010. PMID: 20566851 Free PMC article. - The p75NTR intracellular domain generated by neurotrophin-induced receptor cleavage potentiates Trk signaling.
Ceni C, Kommaddi RP, Thomas R, Vereker E, Liu X, McPherson PS, Ritter B, Barker PA. Ceni C, et al. J Cell Sci. 2010 Jul 1;123(Pt 13):2299-307. doi: 10.1242/jcs.062612. Epub 2010 Jun 8. J Cell Sci. 2010. PMID: 20530577 - Type I interferons up-regulate the expression and signalling of p75 NTR/TrkA receptor complex in differentiated human SH-SY5Y neuroblastoma cells.
Dedoni S, Olianas MC, Ingianni A, Onali P. Dedoni S, et al. Neuropharmacology. 2014 Apr;79:321-34. doi: 10.1016/j.neuropharm.2013.12.002. Epub 2013 Dec 11. Neuropharmacology. 2014. PMID: 24333329 - Roles of p75NTR in the pathogenesis of Alzheimer's disease: a novel therapeutic target.
Zeng F, Lu JJ, Zhou XF, Wang YJ. Zeng F, et al. Biochem Pharmacol. 2011 Nov 15;82(10):1500-9. doi: 10.1016/j.bcp.2011.06.040. Epub 2011 Jul 5. Biochem Pharmacol. 2011. PMID: 21762680 Review. - Drain of the brain: low-affinity p75 neurotrophin receptor affords a molecular sink for clearance of cortical amyloid β by the cholinergic modulator system.
Ovsepian SV, Herms J. Ovsepian SV, et al. Neurobiol Aging. 2013 Nov;34(11):2517-24. doi: 10.1016/j.neurobiolaging.2013.05.005. Epub 2013 Jun 5. Neurobiol Aging. 2013. PMID: 23747048 Review.
Cited by
- New BDNF and NT-3 Cyclic Mimetics Concur with Copper to Activate Trophic Signaling Pathways as Potential Molecular Entities to Protect Old Brains from Neurodegeneration.
Magrì A, Tomasello B, Naletova I, Tabbì G, Cairns WRL, Greco V, Sciuto S, La Mendola D, Rizzarelli E. Magrì A, et al. Biomolecules. 2024 Sep 2;14(9):1104. doi: 10.3390/biom14091104. Biomolecules. 2024. PMID: 39334869 Free PMC article. - The Molecular Pathway of p75 Neurotrophin Receptor (p75NTR) in Parkinson's Disease: The Way of New Inroads.
Ali NH, Al-Kuraishy HM, Al-Gareeb AI, Alnaaim SA, Saad HM, Batiha GE. Ali NH, et al. Mol Neurobiol. 2024 May;61(5):2469-2480. doi: 10.1007/s12035-023-03727-8. Epub 2023 Oct 28. Mol Neurobiol. 2024. PMID: 37897634 Review. - Nerve growth factor receptor (Ngfr) induces neurogenic plasticity by suppressing reactive astroglial Lcn2/Slc22a17 signaling in Alzheimer's disease.
Siddiqui T, Cosacak MI, Popova S, Bhattarai P, Yilmaz E, Lee AJ, Min Y, Wang X, Allen M, İş Ö, Atasavum ZT, Rodriguez-Muela N, Vardarajan BN, Flaherty D, Teich AF, Santa-Maria I, Freudenberg U, Werner C, Tosto G, Mayeux R, Ertekin-Taner N, Kizil C. Siddiqui T, et al. NPJ Regen Med. 2023 Jul 10;8(1):33. doi: 10.1038/s41536-023-00311-5. NPJ Regen Med. 2023. PMID: 37429840 Free PMC article. - Recent Development in the Understanding of Molecular and Cellular Mechanisms Underlying the Etiopathogenesis of Alzheimer's Disease.
Afsar A, Chacon Castro MDC, Soladogun AS, Zhang L. Afsar A, et al. Int J Mol Sci. 2023 Apr 14;24(8):7258. doi: 10.3390/ijms24087258. Int J Mol Sci. 2023. PMID: 37108421 Free PMC article. Review. - Chronic basal forebrain activation improves spatial memory, boosts neurotrophin receptor expression, and lowers BACE1 and Aβ42 levels in the cerebral cortex in mice.
Kumro J, Tripathi A, Lei Y, Sword J, Callahan P, Terry A, Lu XY, Kirov SA, Pillai A, Blake DT. Kumro J, et al. Cereb Cortex. 2023 Jun 8;33(12):7627-7641. doi: 10.1093/cercor/bhad066. Cereb Cortex. 2023. PMID: 36939283 Free PMC article.
References
- De Strooper B., Annaert W. Proteolytic processing and cell biological functions of the amyloid precursor protein. J. Cell Sci. 2000;113:1857–1870. - PubMed
- Puglielli L., Tanzi R. E., Kovacs D. M. Alzheimer's disease: the cholesterol connection. Nat. Neurosci. 2003;6:345–351. - PubMed
- Skovronsky D. M., Lee V. M. β-Secretase revealed: starting gate for race to novel therapies for Alzheimer's disease. Trends Pharmacol. Sci. 2000;21:161–163. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials