AS160, the Akt substrate regulating GLUT4 translocation, has a functional Rab GTPase-activating protein domain - PubMed (original) (raw)
AS160, the Akt substrate regulating GLUT4 translocation, has a functional Rab GTPase-activating protein domain
Cristinel P Mîinea et al. Biochem J. 2005.
Abstract
Recently, we described a 160 kDa protein (designated AS160, for Akt substrate of 160 kDa) with a predicted Rab GAP (GTPase-activating protein) domain that is phosphorylated on multiple sites by the protein kinase Akt. Phosphorylation of AS160 in adipocytes is required for insulin-stimulated translocation of the glucose transporter GLUT4 to the plasma membrane. The aim of the present study was to determine whether AS160 is in fact a GAP for Rabs, and, if so, what its specificity is. We first identified a group of 16 Rabs in a preparation of intracellular vesicles containing GLUT4 by MS. We then prepared the recombinant GAP domain of AS160 and examined its activity against many of these Rabs, as well as several others. The GAP domain was active against Rabs 2A, 8A, 10 and 14. There was no significant activity against 14 other Rabs. GAP activity was further validated by the finding that the recombinant GAP domain with the predicted catalytic arginine residue replaced by lysine was inactive. Finally, it was found by immunoblotting that Rabs 2A, 8A and 14 are present in GLUT4 vesicles. These results indicate that AS160 is a Rab GAP, and suggest novel Rabs that may participate in GLUT4 translocation.
Figures
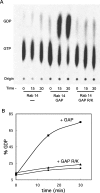
Figure 1. Rab GAP assay with AS160 GST–GAP domain and GST–Rab14
(A) TLC separation of α32P GDP and GTP in assays with Rab14 alone, Rab14 plus the GAP domain, and Rab14 plus the R/K mutant of the GAP domain. (B) The data from (A) are presented as the percentage of the total radioactivity in GDP versus time for Rab14 (●), Rab14 plus the GAP domain (■), and Rab14 plus the R/K mutant GAP domain (▲). For further details, see the Experimental section.
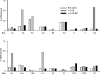
Figure 2. Activity of AS160 GAP domain against the 18 Rab proteins
The GAP assay was carried out as described in the Experimental section and shown in Figure 1. The data show the increase in the percentage of the total radioactivity in GDP between 0 and 15 min for the Rab alone and the Rab plus the AS160 GAP domain. In addition, for any Rab that appeared to be a GAP substrate, as well as most of the others, the assay was also performed with the GAP R/K mutant. In the case of Rab5A, the data are for 3 min, rather than 15 min, due to the high intrinsic GTPase activity of this Rab.
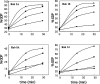
Figure 3. Dependence of the GAP activity upon the concentration of the AS160 GST–GAP domain
The GAP assay was carried out with Rabs 2A, 8A, 10 and 14, as described in the Experimental section and shown in Figure 1. The concentration of the GST–GAP domain in the assay was: none (■), 0.04 μM (◆), 0.13 μM (●) and 0.4 μM (▲).
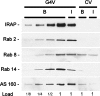
Figure 4. Rabs in GLUT4 vesicles
GLUT4 vesicles (G4V) and control adsorbates (CV) were prepared from unstimulated (B) and insulin-treated (I) 3T3-L1 adipocytes, as described in the Experimental section. SDS samples of the vesicles were immunoblotted for IRAP, Rabs 2, 8 and 14, and AS160. The 1× loads were derived from 0.15% of a 10-cm diameter plate for IRAP, 2% of a plate for Rabs 2, 8 and 14, and 4% of a plate for AS160. Two repetitions of this experiment gave similar results, except for variable relative amounts of Rab14 in the insulin-treated GLUT4 vesicles (see the text).
Similar articles
- Rabs 8A and 14 are targets of the insulin-regulated Rab-GAP AS160 regulating GLUT4 traffic in muscle cells.
Ishikura S, Bilan PJ, Klip A. Ishikura S, et al. Biochem Biophys Res Commun. 2007 Feb 23;353(4):1074-9. doi: 10.1016/j.bbrc.2006.12.140. Epub 2006 Dec 27. Biochem Biophys Res Commun. 2007. PMID: 17208202 - Calmodulin binds to the Rab GTPase activating protein required for insulin-stimulated GLUT4 translocation.
Kane S, Lienhard GE. Kane S, et al. Biochem Biophys Res Commun. 2005 Sep 16;335(1):175-80. doi: 10.1016/j.bbrc.2005.07.056. Biochem Biophys Res Commun. 2005. PMID: 16055084 - Substrate specificity and effect on GLUT4 translocation of the Rab GTPase-activating protein Tbc1d1.
Roach WG, Chavez JA, Mîinea CP, Lienhard GE. Roach WG, et al. Biochem J. 2007 Apr 15;403(2):353-8. doi: 10.1042/BJ20061798. Biochem J. 2007. PMID: 17274760 Free PMC article. - Signal transduction meets vesicle traffic: the software and hardware of GLUT4 translocation.
Klip A, Sun Y, Chiu TT, Foley KP. Klip A, et al. Am J Physiol Cell Physiol. 2014 May 15;306(10):C879-86. doi: 10.1152/ajpcell.00069.2014. Epub 2014 Mar 5. Am J Physiol Cell Physiol. 2014. PMID: 24598362 Review. - Role of Akt substrate of 160 kDa in insulin-stimulated and contraction-stimulated glucose transport.
Cartee GD, Wojtaszewski JF. Cartee GD, et al. Appl Physiol Nutr Metab. 2007 Jun;32(3):557-66. doi: 10.1139/H07-026. Appl Physiol Nutr Metab. 2007. PMID: 17510697 Review.
Cited by
- Smad transcription factors as mediators of 7 transmembrane G protein-coupled receptor signalling.
Chia ZJ, Kumarapperuma H, Zhang R, Little PJ, Kamato D. Chia ZJ, et al. Acta Pharmacol Sin. 2024 Nov 6. doi: 10.1038/s41401-024-01413-6. Online ahead of print. Acta Pharmacol Sin. 2024. PMID: 39506064 Review. - The Interlinking Metabolic Association between Type 2 Diabetes Mellitus and Cancer: Molecular Mechanisms and Therapeutic Insights.
Asiri A, Al Qarni A, Bakillah A. Asiri A, et al. Diagnostics (Basel). 2024 Sep 25;14(19):2132. doi: 10.3390/diagnostics14192132. Diagnostics (Basel). 2024. PMID: 39410536 Free PMC article. Review. - Molecular Aspects in the Development of Type 2 Diabetes and Possible Preventive and Complementary Therapies.
Simon-Szabó L, Lizák B, Sturm G, Somogyi A, Takács I, Németh Z. Simon-Szabó L, et al. Int J Mol Sci. 2024 Aug 22;25(16):9113. doi: 10.3390/ijms25169113. Int J Mol Sci. 2024. PMID: 39201799 Free PMC article. Review. - A comprehensive in-vitro/in-vivo screening toolbox for the elucidation of glucose homeostasis modulating properties of plant extracts (from roots) and its bioactives.
Bauer I, Rimbach G, Cordeiro S, Bosy-Westphal A, Weghuber J, Ipharraguerre IR, Lüersen K. Bauer I, et al. Front Pharmacol. 2024 Jun 26;15:1396292. doi: 10.3389/fphar.2024.1396292. eCollection 2024. Front Pharmacol. 2024. PMID: 38989154 Free PMC article. Review. - Hijacking host cell vesicular transport: New insights into the nutrient acquisition mechanism of Chlamydia.
Wenbo L, Yewei Y, Hui Z, Zhongyu L. Wenbo L, et al. Virulence. 2024 Dec;15(1):2351234. doi: 10.1080/21505594.2024.2351234. Epub 2024 May 21. Virulence. 2024. PMID: 38773735 Free PMC article. Review.
References
- Watson R. T., Kanzaki M., Pessin J. E. Regulated membrane trafficking of the insulin-responsive glucose transporter 4 in adipocytes. Endocrine Rev. 2004;25:177–204. - PubMed
- Bae S. S., Cho H., Mu J., Birnbaum M. J. Isoform-specific regulation of insulin-dependent glucose uptake by Akt/protein B kinase. J. Biol. Chem. 2003;278:49530–49536. - PubMed
- Katome T., Obata T., Matsushima R., Masuyama N., Cantley L. C., Gotoh Y., Kishi K., Shiota H., Ebina Y. Use of RNA interference-mediated gene silencing and adenoviral overexpression to elucidate the roles of Akt/protein kinase B isoforms in insulin actions. J. Biol. Chem. 2003;278:28312–28323. - PubMed
- Kane S., Sano H., Liu S. C. H., Asara J. M., Lane W. S., Garner C. C., Lienhard G. E. A method to identify serine kinase substrates. J. Biol. Chem. 2002;277:22115–22118. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous