Suppression of bladder epithelial cytokine responses by uropathogenic Escherichia coli - PubMed (original) (raw)
Suppression of bladder epithelial cytokine responses by uropathogenic Escherichia coli
David A Hunstad et al. Infect Immun. 2005 Jul.
Abstract
Urinary tract infections are most commonly caused by uropathogenic strains of Escherichia coli (UPEC), which invade superficial bladder epithelial cells via a type 1 pilus-dependent mechanism. Inside these epithelial cells, UPEC organisms multiply to high numbers to form intracellular bacterial communities, allowing them to avoid immune detection. Bladder epithelial cells produce interleukin-6 (IL-6) and IL-8 in response to laboratory strains of E. coli in vitro. We investigated the ability of UPEC to alter epithelial cytokine signaling by examining the in vitro responses of bladder epithelial cell lines to the cystitis strains UTI89 and NU14. The cystitis strains induced significantly less IL-6 than did the laboratory E. coli strain MG1655 from 5637 and T24 bladder epithelial cells. The cystitis strains also suppressed epithelial cytokine responses to exogenous lipopolysaccharide (LPS) and to laboratory E. coli. We found that insertional mutations in the rfa and rfb operons and in the surA gene all abolished the ability of UTI89 to suppress cytokine induction. The rfa and rfb operons encode LPS biosynthetic genes, while surA encodes a periplasmic cis-trans prolyl isomerase important in the biogenesis of outer membrane proteins. We conclude that, in this in vitro model system, cystitis strains of UPEC have genes encoding factors that suppress proinflammatory cytokine production by bladder epithelial cells.
Figures
FIG. 1.
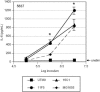
UPEC strains induce less IL-6 from cultured bladder epithelial cells than does E. coli K-12. Confluent bladder cell monolayers were incubated for 2 h with various doses of UPEC strain UTI89 or NU14 or the laboratory E. coli strain MG1655, and IL-6 levels in culture supernatants were measured by sandwich ELISA. (A) IL-6 produced by 5637 cells upon infection with UPEC strain UTI89 or NU14 is equal to unstimulated levels (solid lines), while MG1655 induced significantly more IL-6 at all doses examined (dashed line) (*, P < 0.05; **, P < 0.01). (B) In T24 cells, infection with UPEC induces a modest IL-6 response (solid lines), while stimulation with E. coli K-12 strain MG1655 (dashed line) yields significantly higher IL-6 levels (*, P < 0.03). Experiments were repeated at least three times.
FIG. 2.
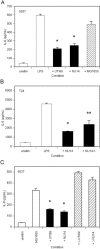
UPEC strains suppress epithelial cytokine responses to exogenously added gram-negative bacterial LPS or E. coli K-12. (A) Confluent monolayers of 5637 cells were left untreated, incubated with 5 μg/ml E. coli O55:B5 LPS, or incubated with LPS plus 107 CFU of the indicated E. coli strains. The UPEC strains UTI89 and NU14 (solid bars) significantly reduced the IL-6 produced in response to exogenous LPS (*, P < 0.01 versus LPS), while MG1655 (striped bar) did not demonstrate such reduction. (B) Confluent monolayers of T24 cells were treated similarly with 500 ng/ml E. coli O55:B5 LPS or with LPSplus 107 CFU of the indicated E. coli strains. The UPEC strain NU14 (*, P < 0.01) and its isogenic fimH mutant NU14-1 (**, P < 0.05) (solid bars) both suppressed T24 cytokine responses to exogenous LPS. (C) Confluent monolayers of 5637 cells were left untreated, treated with 106-CFU/ml MG1655, or treated with MG1655 plus 107 CFU/ml of the indicated UPEC strains. Live UPEC strain UTI89 or NU14 (solid bars) suppressed IL-6 responses to MG1655 (*, P < 0.01 versus MG1655 alone), while heat-killed UTI89 and NU14 (striped bars) have lost this capability. Experiments were repeated at least three times.
FIG. 3.
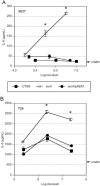
Disruption of LPS biosynthetic operons in UPEC results in cytokine induction from bladder epithelial cells. Confluent monolayers of 5637 cells were treated with the indicated doses of wild-type UTI89 (squares), the transposon insertion mutant 11F5 (circles) or 16C1 (triangles), or the E. coli K-12 strain MG1655 (diamonds; dashed line). At doses of 106 CFU/ml and above, induction of epithelial IL-6 by the LPS mutants 11F5 and 16C1 was significantly higher than that by UTI89 (*, P < 0.05) and approximated the induction by MG1655. Experiments were repeated at least three times.
FIG. 4.
Insertional inactivation of surA results in increased cytokine induction from bladder epithelial cells. Confluent monolayers of 5637 (A) and T24 (B) cells were treated with the indicated doses of wild-type UTI89 (squares), UTI89 surA (open circles), or UTI89 surA/pAER1 (filled circles). The surA mutant displays increased cytokine induction, particularly at doses of 106 CFU/ml and above (*, P < 0.02 versus UTI89), and this phenotype reverts to wild type upon complementation with the plasmid pAER1. Experiments were repeated at least three times.
FIG. 5.
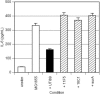
Mutations in LPS biosynthetic genes or in surA abrogate UPEC's ability to suppress cytokine responses to MG1655. Confluent monolayers of 5637 cells were left untreated, treated with 106-CFU/ml MG1655, or treated with MG1655 plus 107 CFU/ml of the indicated UPEC strains. Unlike the UPEC strain UTI89 (solid bar), the LPS biosynthetic mutants 11F5 and 16C1 and the surA mutant (striped bars) fail to suppress epithelial cytokine responses to MG1655. Experiments were repeated at least three times.
Similar articles
- Maturation of intracellular Escherichia coli communities requires SurA.
Justice SS, Lauer SR, Hultgren SJ, Hunstad DA. Justice SS, et al. Infect Immun. 2006 Aug;74(8):4793-800. doi: 10.1128/IAI.00355-06. Infect Immun. 2006. PMID: 16861667 Free PMC article. - Rapid Bladder Interleukin-10 Synthesis in Response to Uropathogenic Escherichia coli Is Part of a Defense Strategy Triggered by the Major Bacterial Flagellar Filament FliC and Contingent on TLR5.
Acharya D, Sullivan MJ, Duell BL, Goh KGK, Katupitiya L, Gosling D, Chamoun MN, Kakkanat A, Chattopadhyay D, Crowley M, Crossman DK, Schembri MA, Ulett GC. Acharya D, et al. mSphere. 2019 Nov 27;4(6):e00545-19. doi: 10.1128/mSphere.00545-19. mSphere. 2019. PMID: 31776239 Free PMC article. - Modulation of host innate immune response in the bladder by uropathogenic Escherichia coli.
Billips BK, Forrestal SG, Rycyk MT, Johnson JR, Klumpp DJ, Schaeffer AJ. Billips BK, et al. Infect Immun. 2007 Nov;75(11):5353-60. doi: 10.1128/IAI.00922-07. Epub 2007 Aug 27. Infect Immun. 2007. PMID: 17724068 Free PMC article. - Bacterial invasion augments epithelial cytokine responses to Escherichia coli through a lipopolysaccharide-dependent mechanism.
Schilling JD, Mulvey MA, Vincent CD, Lorenz RG, Hultgren SJ. Schilling JD, et al. J Immunol. 2001 Jan 15;166(2):1148-55. doi: 10.4049/jimmunol.166.2.1148. J Immunol. 2001. PMID: 11145696 - Molecular basis of uropathogenic Escherichia coli evasion of the innate immune response in the bladder.
Billips BK, Schaeffer AJ, Klumpp DJ. Billips BK, et al. Infect Immun. 2008 Sep;76(9):3891-900. doi: 10.1128/IAI.00069-08. Epub 2008 Jun 16. Infect Immun. 2008. PMID: 18559433 Free PMC article.
Cited by
- Lactobacillus rhamnosus GR-1 enhances NF-kappaB activation in Escherichia coli-stimulated urinary bladder cells through TLR4.
Karlsson M, Scherbak N, Reid G, Jass J. Karlsson M, et al. BMC Microbiol. 2012 Jan 22;12:15. doi: 10.1186/1471-2180-12-15. BMC Microbiol. 2012. PMID: 22264349 Free PMC article. - SurA-like and Skp-like Proteins as Important Virulence Determinants of the Gram Negative Bacterial Pathogens.
Figaj D, Ambroziak P, Rzepka I, Skórko-Glonek J. Figaj D, et al. Int J Mol Sci. 2022 Dec 24;24(1):295. doi: 10.3390/ijms24010295. Int J Mol Sci. 2022. PMID: 36613738 Free PMC article. Review. - Trans-mission control in the urinary tract: Local cytokine regulation of monocyte proliferation to combat infection.
Ligon MM, Mysorekar IU. Ligon MM, et al. J Leukoc Biol. 2018 Jan;103(1):5-7. doi: 10.1002/JLB.4CE0917-382R. Epub 2017 Dec 11. J Leukoc Biol. 2018. PMID: 29345062 Free PMC article. - Urothelial generation and regeneration in development, injury, and cancer.
Wang C, Ross WT, Mysorekar IU. Wang C, et al. Dev Dyn. 2017 Apr;246(4):336-343. doi: 10.1002/dvdy.24487. Epub 2017 Mar 2. Dev Dyn. 2017. PMID: 28109014 Free PMC article. Review. - Subversion of Host Innate Immunity by Uropathogenic Escherichia coli.
Olson PD, Hunstad DA. Olson PD, et al. Pathogens. 2016 Jan 4;5(1):2. doi: 10.3390/pathogens5010002. Pathogens. 2016. PMID: 26742078 Free PMC article. Review.
References
- Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499-511. - PubMed
- Bäckhed, F., S. Normark, E. K. Schweda, S. Oscarson, and A. Richter-Dahlfors. 2003. Structural requirements for TLR4-mediated LPS signalling: a biological role for LPS modifications. Microbes Infect. 5:1057-1063. - PubMed
- Bäckhed, F., M. Soderhall, P. Ekman, S. Normark, and A. Richter-Dahlfors. 2001. Induction of innate immune responses by Escherichia coli and purified lipopolysaccharide correlate with organ- and cell-specific expression of Toll-like receptors within the human urinary tract. Cell. Microbiol. 3:153-158. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01-DK51406/DK/NIDDK NIH HHS/United States
- P50-DK64540/DK/NIDDK NIH HHS/United States
- F32 DK010168/DK/NIDDK NIH HHS/United States
- P50 DK064540/DK/NIDDK NIH HHS/United States
- K08 DK067894/DK/NIDDK NIH HHS/United States
- R01 DK051406/DK/NIDDK NIH HHS/United States
- K12 HD000850/HD/NICHD NIH HHS/United States
- K12-HD00850/HD/NICHD NIH HHS/United States
- K08-DK067894/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources