Enteropathogenic Escherichia coli type III effectors EspG and EspG2 disrupt the microtubule network of intestinal epithelial cells - PubMed (original) (raw)
Enteropathogenic Escherichia coli type III effectors EspG and EspG2 disrupt the microtubule network of intestinal epithelial cells
Robert K Shaw et al. Infect Immun. 2005 Jul.
Abstract
Enteropathogenic Escherichia coli infection of intestinal epithelial cells leads to localized depletion of the microtubule cytoskeleton, an effect that is dependent on delivery of type III translocated effector proteins EspG and Orf3 (designated EspG2) to the site of depletion. Microtubule depletion involved disruption rather than displacement of microtubules.
Figures
FIG. 1.
Translocation of EPEC effector proteins EspG and Orf3 into live HeLa cells using TEM-1 fusion and fluorescence microscopy. HeLa cells were infected with E2348/69 expressing pCX327 (positive control) (a), pCX340 (negative control) (b), pICC305 (c), or pICC306 (d) or with EPEC escF expressing pICC305 (e) or pICC306 (f). Blue fluorescence demonstrates translocation of EspG and Orf3 when expressed in the E2348/69 background (c, d), whereas green fluorescence indicates lack of translocation when expressed from the type III secretion escF mutant (e, f).
FIG. 2.
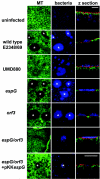
Confocal images showing uninfected Caco-2 cell monolayers (row 1); Caco-2 cells infected for 1 h with primed cultures of E2348/69 (row 2), type III secretion mutant UMD880 (row 3), the espG mutant (row 4), the orf3 mutant (row 5), and the espG orf3 double mutant (row 6); and cells infected for 3 h with an unprimed culture of the espG orf3 double mutant complemented with cloned espG. Cells were stained for microtubules (MT; column 1) and bacteria (column 2). Columns 1 and 2 are maximum projections; column 3 shows transverse projections through cell monolayers that have also been stained for cellular actin (red). E2348/69-, _espG_-, and _orf3_-infected cells reveal microtubule depletion (column 1, asterisks) beneath adherent bacterial microcolonies (column 2, asterisks) but not in cells infected with UMD880 or the espG orf3 double mutant. Microtubule depletion was restored with the espG orf3 double mutant possessing cloned espG. Bars, 20 μm.
FIG. 3.
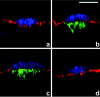
Confocal image projections through Caco-2 cell monolayers infected for 1 h with wild-type E2348/69 (a), E2348/69(pICC307) (b), espG(pICC307) (c), and escF(pICC307) (d) and stained for HA (green), actin (red), and bacteria (blue). EspG-HA is translocated into Caco-2 cells by E2348/69(pICC307) (b) and espG(pICC307) (c) and is localized beneath bacterial microcolonies. EspG-HA is not translocated by the type III mutant escF(pICC307) (d). Bar, 10 μm.
FIG. 4.
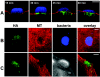
Confocal images of Caco-2 cells (A, B) and HEp-2 cells (C) infected with E2348/69(pICC307). Panel A, cells infected for 15, 30, 45, and 60 min and stained for HA (green), actin (red), and bacteria (blue). Translocated EspG-HA is detectable in cells only after 30 min and then continuously up to 1 h. Panels B and C, cells infected for 30 min and stained for HA (green) and microtubules (MT; red). In Caco-2 cells, bacteria were stained blue. In HEp-2 cells, bacteria were visualized by phase contrast. Both panels show translocated EspG-HA beneath adherent bacterial microcolonies colocalized with areas of microtubule depletion. Microtubules appear to be disrupted rather than displaced. Bars: A and C, 10 μm; B, 20 μm.
FIG. 5.
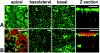
Confocal images showing uninfected Caco-2 cells (A) and Caco-2 cells infected for 1 h with wild-type E2348/69 (B). Cells were stained for actin (green) and bacteria (red), and image sections show the apical, basolateral, and basal regions of cells and z projections through the cell monolayer. Caco-2 cells possess an apical brush border rich in actin and membrane-associated basolateral and basal actin (A). E2348/69 infection results in bacterial adhesion and actin accretion at the apical brush border surface of Caco-2 cells but no detectable changes in basolateral and basal cell actin (B). Bars, 10 μm.
Similar articles
- Enteropathogenic E. coli effectors EspG1/G2 disrupt tight junctions: new roles and mechanisms.
Glotfelty LG, Hecht GA. Glotfelty LG, et al. Ann N Y Acad Sci. 2012 Jul;1258:149-58. doi: 10.1111/j.1749-6632.2012.06563.x. Ann N Y Acad Sci. 2012. PMID: 22731728 Free PMC article. Review. - Function and distribution of EspG2, a type III secretion system effector of enteropathogenic Escherichia coli.
Smollett K, Shaw RK, Garmendia J, Knutton S, Frankel G. Smollett K, et al. Microbes Infect. 2006 Jul;8(8):2220-7. doi: 10.1016/j.micinf.2006.04.004. Epub 2006 May 24. Microbes Infect. 2006. PMID: 16781180 - Enteropathogenic Escherichia coli EspG disrupts microtubules and in conjunction with Orf3 enhances perturbation of the tight junction barrier.
Tomson FL, Viswanathan VK, Kanack KJ, Kanteti RP, Straub KV, Menet M, Kaper JB, Hecht G. Tomson FL, et al. Mol Microbiol. 2005 Apr;56(2):447-64. doi: 10.1111/j.1365-2958.2005.04571.x. Mol Microbiol. 2005. PMID: 15813736 - Enteropathogenic Escherichia coli type III effectors EspG and EspG2 alter epithelial paracellular permeability.
Matsuzawa T, Kuwae A, Abe A. Matsuzawa T, et al. Infect Immun. 2005 Oct;73(10):6283-9. doi: 10.1128/IAI.73.10.6283-6289.2005. Infect Immun. 2005. PMID: 16177299 Free PMC article. - Subversion of actin dynamics by EPEC and EHEC.
Caron E, Crepin VF, Simpson N, Knutton S, Garmendia J, Frankel G. Caron E, et al. Curr Opin Microbiol. 2006 Feb;9(1):40-5. doi: 10.1016/j.mib.2005.12.008. Epub 2006 Jan 6. Curr Opin Microbiol. 2006. PMID: 16406772 Review.
Cited by
- Sequence capture and next generation resequencing of the MHC region highlights potential transplantation determinants in HLA identical haematopoietic stem cell transplantation.
Pröll J, Danzer M, Stabentheiner S, Niklas N, Hackl C, Hofer K, Atzmüller S, Hufnagl P, Gülly C, Hauser H, Krieger O, Gabriel C. Pröll J, et al. DNA Res. 2011 Aug;18(4):201-10. doi: 10.1093/dnares/dsr008. Epub 2011 May 28. DNA Res. 2011. PMID: 21622977 Free PMC article. - Comparative Genomics of Atypical Enteropathogenic Escherichia coli from Kittens and Children Identifies Bacterial Factors Associated with Virulence in Kittens.
Watson VE, Hazen TH, Rasko DA, Jacob ME, Elfenbein JR, Stauffer SH, Gookin JL. Watson VE, et al. Infect Immun. 2021 Feb 16;89(3):e00619-20. doi: 10.1128/IAI.00619-20. Print 2021 Feb 16. Infect Immun. 2021. PMID: 33257534 Free PMC article. - EspF of enteropathogenic Escherichia coli binds sorting nexin 9.
Marchès O, Batchelor M, Shaw RK, Patel A, Cummings N, Nagai T, Sasakawa C, Carlsson SR, Lundmark R, Cougoule C, Caron E, Knutton S, Connerton I, Frankel G. Marchès O, et al. J Bacteriol. 2006 Apr;188(8):3110-5. doi: 10.1128/JB.188.8.3110-3115.2006. J Bacteriol. 2006. PMID: 16585770 Free PMC article. - Enteropathogenic E. coli effectors EspG1/G2 disrupt tight junctions: new roles and mechanisms.
Glotfelty LG, Hecht GA. Glotfelty LG, et al. Ann N Y Acad Sci. 2012 Jul;1258:149-58. doi: 10.1111/j.1749-6632.2012.06563.x. Ann N Y Acad Sci. 2012. PMID: 22731728 Free PMC article. Review. - Enteropathogenic and enterohemorrhagic Escherichia coli infections: translocation, translocation, translocation.
Garmendia J, Frankel G, Crepin VF. Garmendia J, et al. Infect Immun. 2005 May;73(5):2573-85. doi: 10.1128/IAI.73.5.2573-2585.2005. Infect Immun. 2005. PMID: 15845459 Free PMC article. Review. No abstract available.
References
- Campellone, K. G., D. Robbins, and J. M. Leong. 2004. EspFU is a translocated EHEC effector that interacts with Tir and N-WASP and promotes Nck-dependent actin assembly. Dev. Cell 7:217-228. - PubMed
- Cleary, J., L.-C. Lai, R. K Shaw, A. Straatman-Iwanowska, M. S. Donnenberg, G. Frankel, and S. Knutton. 2004. Enteropathogenic Escherichia coli (EPEC) adhesion to intestinal epithelial cells: role of bundle-forming pili (BFP), EspA filaments and intimin. Microbiology 150:527-538. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources