Age-related loss of synaptophysin immunoreactive presynaptic boutons within the hippocampus of APP751SL, PS1M146L, and APP751SL/PS1M146L transgenic mice - PubMed (original) (raw)
Age-related loss of synaptophysin immunoreactive presynaptic boutons within the hippocampus of APP751SL, PS1M146L, and APP751SL/PS1M146L transgenic mice
Bart P F Rutten et al. Am J Pathol. 2005 Jul.
Abstract
Neuron and synapse loss are important features of the neuropathology of Alzheimer's disease (AD). Recently, we observed substantial age-related hippocampal neuron loss in APP751SL/PS1M146L transgenic mice but not in PS1M146L mice. Here, we investigated APP751SL mice, PS1M146L mice, and APP751SL/PS1M146L mice for age-related alterations in synaptic integrity within hippocampal stratum moleculare of the dentate gyrus (SM), stratum lucidum of area CA3 (SL), and stratum radiatum of area CA1-2 (SR) by analyzing densities and numbers of synaptophysin-immunoreactive presynaptic boutons (SIPBs). Wild-type mice, APP751SL mice and PS1M146L mice showed similar amounts of age-related SIPB loss within SM, and no SIPB loss within SL. Both APP751SL mice and PS1M146L mice showed age-related SIPB loss within SR. Importantly, APP751SL/PS1M146L) mice displayed the severest age-related SIPB loss within SM, SL, and SR, even in regions free of extracellular Abeta deposits. Together, these mouse models offer a unique framework to study the impact of several molecular and cellular events caused by mutant APP and/or mutant PS1 on age-related alterations in synaptic integrity. The observation of age-related SIPB loss within SR of PS1M146L mice supports a role of mutant PS1 in neurodegeneration apart from its contribution to alterations in Abeta generation.
Figures
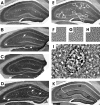
Figure 1
Representative photomicrographs of frontal sections through the hippocampus of a 17-month-old wild-type control mouse (A, E to H, and K), a 17-month-old APP751SL mouse (B), a 17-month-old PS1M146L mouse, and a 17-month-old APP751SL/PS1M146L mouse (D and I), showing immunoreactivity for synaptophysin. Aβ deposits within the hippocampus of the APP751SL mouse and the APP751SL/PS1M146L mouse are indicated by arrows in B and D, respectively. The small squares in E represent the areas where the high-power photomicrographs for quantitative SIPB analysis were taken within SM, SL, and SR. Corresponding high-power photomicrographs depict larger SIPBs within SL (G) than within SM (F) and SR (H). Aβ deposits were clearly recognizable as round/oval structures not immunoreactive for synaptophysin and surrounded by multiple enlarged SIPBs and disturbed neuropil (I). The dotted line in I indicates the delineation of Aβ deposits as performed for the analysis of SIPB densities in regions free of Aβ deposits and Aβ aggregation-related disturbances in normal SIPB morphology. In K, the projection areas of SM, SL, and SR are shown. Scale bar = 250 μm in A to E and K, 20 μm in F to H, and 10 μm in I.
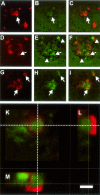
Figure 2
Representative high-power photomicrographs obtained by TPLSM, showing alterations in synaptic integrity caused by Aβ deposits within hippocampal subregion SR of a 17-month-old APP751SL/PS1M146L mouse. Aβ immunoreactivity is shown in red in A, D, and G; synaptophysin immunoreactivity at the corresponding locations and focal planes in green in B, E, and H; and merged pictures in C, F, and I. Note the disturbed SIPB morphology within and in the near vicinity of the Aβ deposits (arrows). The SIPB-free regions around the Aβ deposits (arrowheads in E and F) are most probably caused by astrocytes surrounding the Aβ deposits. Interestingly, several enlarged SIPBs were entirely surrounded by β-amyloid (arrows in I). In K to M, a three-dimensional reconstruction of a representative image stack acquired with TPLSM is shown, displaying synaptophysin (green) and Aβ (red) immunoreactivity within SR of a 17-month-old APP751SL/PS1M146L mouse. The dotted lines in K represent the position within the X-Y view at which the Y-Z view (L) and the X-Z view (M) were generated. The depicted enlarged synaptophysin-immunoreactive presynaptic bouton is surrounded by a rim of Aβ immunoreactivity. Scale bar = 100 μm in A to C, 50 μm in D to I, and 5 μm in K to M.
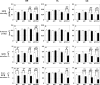
Figure 3
Results of the quantitative investigations in the hippocampus. Analysis of hippocampal SM (A, D, G, and K), SL (B, E, H, and L), and SL (C, F, I, M) of wild-type control mice (WT), APP751SL mice (APP), PS1M146L mice (PS1), and APP751SL/PS1M146L mice (APP/PS1) for age-related alterations in SIPB densities within regions free of Aβ deposits and Aβ aggregation-related disturbances in normal SIPB morphology (A to C); volumes of SM, SL, and SR (D to F); SIPB numbers within regions free of Aβ deposits and Aβ aggregation-related disturbances in normal SIPB morphology (G to I); and corrected SIPB numbers considering the amount of Aβ deposits within SM, SL, and SR (K to M). Results of 4.5-month-old mice (M4.5) are represented by open bars; those of 17-month-old mice (M17) by closed bars. Data are given as mean and SEM. Comparisons between the groups were performed separately for SM, SL, and SR with two-way ANOVA (P values summarized in Table 1) followed by Bonferroni posttests to compare replicate means focusing on age-related effects (P values provided as *P < 0.05, **P < 0.01, or ***P < 0.001 in A, B, C, D, G, H, I, K, L, and M). Significant differences in mean data are indicated (M17 versus M4.5) above each graph. ROI, region of interest; Corr., corrected. SIPB numbers were calculated by multiplying the individual SIPB density data with the corresponding volume data not considering the dimensions (see Materials and Methods for details).
Similar articles
- The impact of Abeta-plaques on cortical cholinergic and non-cholinergic presynaptic boutons in alzheimer's disease-like transgenic mice.
Hu L, Wong TP, Côté SL, Bell KF, Cuello AC. Hu L, et al. Neuroscience. 2003;121(2):421-32. doi: 10.1016/s0306-4522(03)00394-4. Neuroscience. 2003. PMID: 14522000 - Early neuronal loss and axonal/presynaptic damage is associated with accelerated amyloid-β accumulation in AβPP/PS1 Alzheimer's disease mice subiculum.
Trujillo-Estrada L, Dávila JC, Sánchez-Mejias E, Sánchez-Varo R, Gomez-Arboledas A, Vizuete M, Vitorica J, Gutiérrez A. Trujillo-Estrada L, et al. J Alzheimers Dis. 2014;42(2):521-41. doi: 10.3233/JAD-140495. J Alzheimers Dis. 2014. PMID: 24927710 - Neuropathology of mice carrying mutant APP(swe) and/or PS1(M146L) transgenes: alterations in the p75(NTR) cholinergic basal forebrain septohippocampal pathway.
Jaffar S, Counts SE, Ma SY, Dadko E, Gordon MN, Morgan D, Mufson EJ. Jaffar S, et al. Exp Neurol. 2001 Aug;170(2):227-43. doi: 10.1006/exnr.2001.7710. Exp Neurol. 2001. PMID: 11476589 - Neocortical synaptic bouton number is maintained despite robust amyloid deposition in APP23 transgenic mice.
Boncristiano S, Calhoun ME, Howard V, Bondolfi L, Kaeser SA, Wiederhold KH, Staufenbiel M, Jucker M. Boncristiano S, et al. Neurobiol Aging. 2005 May;26(5):607-13. doi: 10.1016/j.neurobiolaging.2004.06.010. Neurobiol Aging. 2005. PMID: 15708435 - Age-related amyloid beta deposition in transgenic mice overexpressing both Alzheimer mutant presenilin 1 and amyloid beta precursor protein Swedish mutant is not associated with global neuronal loss.
Takeuchi A, Irizarry MC, Duff K, Saido TC, Hsiao Ashe K, Hasegawa M, Mann DM, Hyman BT, Iwatsubo T. Takeuchi A, et al. Am J Pathol. 2000 Jul;157(1):331-9. doi: 10.1016/s0002-9440(10)64544-0. Am J Pathol. 2000. PMID: 10880403 Free PMC article.
Cited by
- Hippocampal interneuron loss in an APP/PS1 double mutant mouse and in Alzheimer's disease.
Takahashi H, Brasnjevic I, Rutten BP, Van Der Kolk N, Perl DP, Bouras C, Steinbusch HW, Schmitz C, Hof PR, Dickstein DL. Takahashi H, et al. Brain Struct Funct. 2010 Mar;214(2-3):145-60. doi: 10.1007/s00429-010-0242-4. Epub 2010 Mar 7. Brain Struct Funct. 2010. PMID: 20213270 Free PMC article. - Effects of donepezil on amyloid-beta and synapse density in the Tg2576 mouse model of Alzheimer's disease.
Dong H, Yuede CM, Coughlan CA, Murphy KM, Csernansky JG. Dong H, et al. Brain Res. 2009 Dec 15;1303:169-78. doi: 10.1016/j.brainres.2009.09.097. Epub 2009 Sep 30. Brain Res. 2009. PMID: 19799879 Free PMC article. - Downregulation of caveolin-1 contributes to the synaptic plasticity deficit in the hippocampus of aged rats.
Liu Y, Liang Z, Liu J, Zou W, Li X, Wang Y, An L. Liu Y, et al. Neural Regen Res. 2013 Oct 15;8(29):2725-33. doi: 10.3969/j.issn.1673-5374.2013.29.004. Neural Regen Res. 2013. PMID: 25206583 Free PMC article. - Effects of diet on brain plasticity in animal and human studies: mind the gap.
Murphy T, Dias GP, Thuret S. Murphy T, et al. Neural Plast. 2014;2014:563160. doi: 10.1155/2014/563160. Epub 2014 May 12. Neural Plast. 2014. PMID: 24900924 Free PMC article. Review. - Gene expression parallels synaptic excitability and plasticity changes in Alzheimer's disease.
Saura CA, Parra-Damas A, Enriquez-Barreto L. Saura CA, et al. Front Cell Neurosci. 2015 Aug 25;9:318. doi: 10.3389/fncel.2015.00318. eCollection 2015. Front Cell Neurosci. 2015. PMID: 26379494 Free PMC article. Review.
References
- Cummings JL. Alzheimer’s disease. N Engl J Med. 2004;351:56–67. - PubMed
- Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–791. - PubMed
- Morrison JH, Hof PR. Selective vulnerability of corticocortical and hippocampal circuits in aging and Alzheimer’s disease. Prog Brain Res. 2002;136:467–486. - PubMed
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. - PubMed
- Scheff SW, Price DA. Synaptic pathology in Alzheimer’s disease: a review of ultrastructural studies. Neurobiol Aging. 2003;24:1029–1046. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous