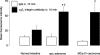Uniform overexpression and rapid accessibility of alpha5beta1 integrin on blood vessels in tumors - PubMed (original) (raw)
Comparative Study
Uniform overexpression and rapid accessibility of alpha5beta1 integrin on blood vessels in tumors
Patricia Parsons-Wingerter et al. Am J Pathol. 2005 Jul.
Abstract
Integrin alpha5beta1 is among the proteins overexpressed on tumor vessels and is a potential target for diagnostics and therapeutics. Here, we mapped the distribution of alpha5beta1 integrin in three murine tumor models and identified sites of expression that are rapidly accessible to intravascular antibodies. When examined by conventional immunohistochemistry, alpha5beta1 integrin expression was strong on most blood vessels in RIP-Tag2 transgenic mouse tumors, adenomatous polyposis coli (apc) mouse adenomas, and implanted MCa-IV mammary carcinomas. Expression increased during malignant progression in RIP-Tag2 mice. However, immunoreactivity was also strong in normal pancreatic ducts, intestinal smooth muscle, and several other sites. To determine which sites of expression were rapidly accessible from the bloodstream, we intravenously injected anti-alpha5beta1 integrin antibody and 10 minutes to 24 hours later examined the amount and distribution of labeling. The injected antibody strongly labeled tumor vessels at all time points but did not label most normal blood vessels or gain access to pancreatic ducts or intestinal smooth muscle. Intense vascular labeling by anti-alpha5beta1 integrin antibody co-localized with the uniform CD31 immunoreactivity of tumor vessels and contrasted sharply with the patchy accumulation of nonspecific IgG at sites of leakage. This strategy of injecting antibodies revealed the uniform overexpression and rapid accessibility of alpha5beta1 integrin on tumor vessels and may prove useful in assessing other potential therapeutic targets in cancer.
Figures
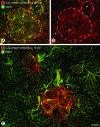
Figure 1
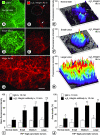
Rapidly accessible α5β1 integrin on blood vessels of RIP-Tag2 tumors. Confocal microscopic images showing α5β1 integrin immunoreactivity (red) and CD31 immunoreactivity (green) in pancreas of RIP-Tag2 mice. A and B: Blood vessels (arrows) in tumors (outlined) and pancreatic ducts (arrowheads) both have strong α5β1 integrin immunoreactivity after staining by conventional immunohistochemistry. A: However, co-localization of CD31 (yellow-green) immunoreactivity co-localizes with α5β1 integrin on tumor vessels but not on ducts (red) or normal blood vessels (green) of the acinar pancreas. C: At 10 minutes after intravenous injection of anti-α5β1 integrin antibody, tumor vessels (arrows), but not pancreatic ducts or normal acinar vessels, have strong immunoreactivity. C: Normal-sized islet (arrowhead) in RIP-Tag2 mouse shows minimal binding of α5β1 integrin antibody. Scale bar, 100 μm (A, B); 75 μm (C).
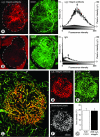
Figure 2
Amount and distribution of antibodies in tumors. Pairs of fluorescence microscopic images of RIP-Tag2 tumors fixed 10 minutes after intravenous injection of anti-α5β1 integrin antibody (A, left) or nonspecific IgG (B, left). Both sections are also stained for CD31 immunoreactivity (right). Dashed lines mark tumor perimeter and define the ROI used for fluorescence measurements. In graphs of A and B, lower histogram (solid) shows number of pixels at fluorescence intensities ranging from 0 (black) to 255 (white) in the ROI of a grayscale version of the corresponding red-fluorescent image (y axis, left). Upper histogram (open) of each graph shows distribution of weighted intensities calculated by multiplying pixel frequencies by their respective fluorescence intensities (y axis, right). Arrows mark arithmetic means of weighted intensities. C: Confocal micrograph of tumor in RIP-Tag2 mouse at 10 minutes after intravenous injection of anti-CD31 (green) and anti-α5β1 integrin antibodies (red). Most blood vessels within the central round tumor are labeled by both antibodies (yellow-orange), but those in the surrounding acinar pancreas are labeled only by the anti-CD31 antibody (arrows). D–F: Binary images of C that show the distribution of anti-α5β1 integrin (D), anti-CD31 (E) pixels above a threshold intensity of 60, and the pixels where the two antibodies co-localize (F, white). Arrows in E mark vessels in acinar pancreas. In this case, 85% of α5β1 integrin pixels co-localize with CD31 pixels. G: Bar graph showing the area densities of anti-CD31 and anti-α5β1 integrin antibodies in RIP-Tag2 tumors 10 minutes after injection (20 images of 20-μm sections of tumors from three mice, threshold = 35). Scale bar, 100 μm (A, B, D–F); 50 μm (C).
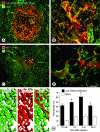
Figure 3
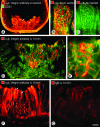
Rapid accumulation of anti-α5β1 integrin antibody versus patchy leakage of nonspecific IgG in RIP-Tag2 tumors. A–D: Confocal microscopic images showing distribution of intravenously injected anti-α5β1 integrin antibody or nonspecific IgG (red) in relation to CD31-positive blood vessels (green) in RIP-Tag2 tumors. A: Most immunoreactivity of anti-α5β1 integrin antibody co-localizes with CD31 staining of blood vessels within the tumor (yellow-orange) but not with normal vessels (green) outside the tumor. B: At higher magnification, anti-α5β1 integrin antibody can be seen to label blood vessels (yellow-orange, arrows) and accumulate in scattered patches of extravasation (red, arrowheads) in tumors. C and D: By comparison, immunoreactivity for nonspecific IgG is patchy, does not co-localize with CD31-positive tumor vessels, and is primarily extravascular. E–G: Schematic representation comparing the uniform labeling of RIP-Tag2 tumor vessels by anti-CD31 (E, green) and anti-α5β1 integrin (F, red) antibodies with the patchy extravasation of nonspecific IgG (G, red) without vessel labeling. H: Bar graph showing the kinetics of accumulation of injected anti-α5β1 integrin antibody and nonspecific IgG in RIP-Tag2 tumors. Anti-integrin antibody accumulated faster and in greater amounts than IgG at all time points (*P < 0.05). Scale bar, 100 μm (A, C); 50 μm (B, D).
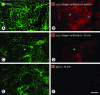
Figure 4
Weak α5β1 integrin immunoreactivity of normal islet blood vessels. Confocal microscopic images showing immunoreactivity of CD31 (green) and α5β1 integrin (red) in normal pancreatic islets. When stained for α5β1 integrin by conventional immunohistochemistry, blood vessels of islets have weak immunoreactivity (A and B, arrow), and ducts of pancreatic acini have strong immunoreactivity (B, arrowhead). C and D: After injection of anti-α5β1 integrin antibody, islet blood vessels have weak immunoreactivity (arrow) and ducts have little or none. E and F: After injection of nonspecific IgG, normal islets and acini have little or no staining for IgG. Scale bar, 50 μm.
Figure 5
Increasing binding of intravenously injected anti-α5β1 integrin antibody during tumor progression. Fluorescence micrographs and corresponding surface plots of tissue fluorescence showing islet in wild-type mouse (A–C, dashed circle) and small tumor (D–F) and large tumor (G–H) in RIP-Tag2 mice. CD31 stained on-section by conventional immunohistochemistry (green). Immunoreactivity of anti-α5β1 integrin antibody at 10 minutes after intravenous injection (red). Height of peaks in surface plots indicates intensity of immunofluorescence. Number of peaks reflects vascularity. I: Bar graph showing intensity of antibody fluorescence at 10 minutes after intravenous injection of anti-α5β1 integrin antibody or nonspecific IgG in RIP-Tag2 mice. After intravenous injection of anti-α5β1 integrin antibody, the immunoreactivity is greater in RIP-Tag2 tumors than in normal islets. This difference increases during tumor progression, as reflected by increasing tumor size. Fluorescence of extravasated nonspecific IgG tends to increase with increasing tumor size, presumably reflecting increased extravasation during tumor progression. However, fluorescence from nonspecific IgG is consistently less than corresponding values for anti-α5β1 integrin antibody. J: Area densities of nonspecific IgG and anti-α5β1 integrin antibody immunofluorescence in the same tumors as I reflect tumor vascularity. Vascularity is significantly greater in tumors than in normal islets, but the values are about the same for tumors of different size. Mean ± SE; n = 4 mice per group. *P < 0.05 for anti-α5β1 integrin antibody compared to nonspecific IgG; †P < 0.05 for tumors compared to normal islets; §P < 0.05 for large tumors compared to small tumors. Scale bar,150 μm.
Figure 6
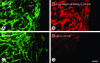
Rapidly accessible α5β1 integrin on blood vessels of intestinal adenomas in apc mice. Fluorescence microscopic images showing α5β1 integrin immunoreactivity (red) and CD31 immunoreactivity of blood vessels (green) in adenomas and normal intestine of apc mice. A: Strong α5β1 integrin immunoreactivity is evident in intestinal adenoma (arrows) and smooth muscle of the intestinal wall (arrowheads) after conventional on-section immunohistochemical staining. B: Smooth muscle cells (arrowheads) of normal intestinal villi also have strong immunoreactivity. At 10 minutes after intravenous injection of anti-α5β1 integrin antibody, neither smooth muscle cells nor blood vessels of normal intestinal villi have immunoreactivity (C), but blood vessels (arrow) in adenomas have strong staining (D). E: Blood vessel staining in adenomas has fuzzy borders suggestive of antibody leakage and binding to perivascular cells. F: Diffuse staining (arrows) is greatest in the apical portion of adenomas. G: By comparison, adenomas have much fainter and more patchy staining after injection of nonspecific IgG, consistent with labeling of extravasated IgG rather than blood vessels. Scale bar, 200 μm (A); 10 μm (B, C, E); 150 μm (D); 100 μm (F, G).
Figure 7
Immunofluorescence of intravenously injected anti-α5β1 integrin antibody in apc adenomas and MCa-IV carcinomas. Bar graph comparing immunofluorescence intensity at 10 minutes after intravenous injection of anti-α5β1 integrin antibody or nonspecific IgG in normal intestine, intestinal adenomas in apc mice, and implanted MCa-IV mammary carcinomas. After intravenous injection of anti-α5β1 integrin antibody, antibody fluorescence is greater in apc tumors than in normal intestine. Both apc adenomas and MCa-IV carcinomas have significantly greater staining for anti-α5β1 integrin antibody than for nonspecific IgG. Mean ± SE; n = 4 mice per group (n = 3 for nonspecific IgG in apc adenomas). *P < 0.05 compared to nonspecific IgG; †P < 0.05 compared to corresponding value for normal intestine.
Figure 8
Rapidly accessible α5β1 integrin on blood vessels of MCa-IV mammary carcinomas. Fluorescence micrographs showing CD31-positive blood vessels (A, C; green) and comparing immunoreactivity (red) for injected anti-α5β1 integrin antibody or nonspecific IgG in MCa-IV mammary carcinomas. At 10 minutes after intravenous injection of anti-α5β1 integrin antibody, tumor vessels have uniform staining (B), but after injection of nonspecific IgG, staining is faint and patchy, and does not co-localize with tumor vessels (D). Scale bar, 100 μm.
Figure 9
Amount and accessibility of α5β1 integrin in normal organs. Fluorescence micrographs of normal organs and tumors in RIP-Tag2 mice comparing amounts of immunoreactivity at 10 minutes after intravenous injection of anti-α5β1 integrin antibody or nonspecific IgG. All images have various shades of gold because they were obtained with standardized settings of the digital camera optimized for the yellow-orange Cy3 fluorophore attached to the secondary antibody. With these camera settings, no staining is visible in brain after intravenous injection of anti-α5β1 integrin antibody (A) or nonspecific IgG (B). Liver sinusoids have strong immunoreactivity after intravenous injection of anti-α5β1 integrin antibody (C, left), and after conventional immunohistochemistry for α5β1 integrin where the antibody is put on the section (C, right), but not after nonspecific IgG (D). For comparison, RIP-Tag2 tumors are shown after injection of anti-α5β1 integrin antibody (E) or nonspecific IgG (F). High endothelial venules (arrow) in mesenteric lymph node have strong immunoreactivity after injection of anti-α5β1 integrin antibody (G) but not after nonspecific IgG (H). Scale bar, 100 μm.
Similar articles
- Rapid access of antibodies to alpha5beta1 integrin overexpressed on the luminal surface of tumor blood vessels.
Magnussen A, Kasman IM, Norberg S, Baluk P, Murray R, McDonald DM. Magnussen A, et al. Cancer Res. 2005 Apr 1;65(7):2712-21. doi: 10.1158/0008-5472.CAN-04-2691. Cancer Res. 2005. PMID: 15805270 - Antiangiogenic therapy decreases integrin expression in normalized tumor blood vessels.
Yao VJ, Ozawa MG, Varner AS, Kasman IM, Chanthery YH, Pasqualini R, Arap W, McDonald DM. Yao VJ, et al. Cancer Res. 2006 Mar 1;66(5):2639-49. doi: 10.1158/0008-5472.CAN-05-1824. Cancer Res. 2006. PMID: 16510583 - Complementary, Selective PET Imaging of Integrin Subtypes α5β1 and αvβ3 Using 68Ga-Aquibeprin and 68Ga-Avebetrin.
Notni J, Steiger K, Hoffmann F, Reich D, Kapp TG, Rechenmacher F, Neubauer S, Kessler H, Wester HJ. Notni J, et al. J Nucl Med. 2016 Mar;57(3):460-6. doi: 10.2967/jnumed.115.165720. Epub 2015 Dec 3. J Nucl Med. 2016. PMID: 26635338 - Probing the structural and molecular diversity of tumor vasculature.
Pasqualini R, Arap W, McDonald DM. Pasqualini R, et al. Trends Mol Med. 2002 Dec;8(12):563-71. doi: 10.1016/s1471-4914(02)02429-2. Trends Mol Med. 2002. PMID: 12470989 Review. - Targeting integrin α5β1 in urological tumors: opportunities and challenges.
Zhou X, Zhu H, Luo C, Xiao H, Zou X, Zou J, Zhang G. Zhou X, et al. Front Oncol. 2023 Jul 6;13:1165073. doi: 10.3389/fonc.2023.1165073. eCollection 2023. Front Oncol. 2023. PMID: 37483505 Free PMC article. Review.
Cited by
- Positive and Negative Regulation of Angiogenesis by Soluble Vascular Endothelial Growth Factor Receptor-1.
Failla CM, Carbo M, Morea V. Failla CM, et al. Int J Mol Sci. 2018 Apr 27;19(5):1306. doi: 10.3390/ijms19051306. Int J Mol Sci. 2018. PMID: 29702562 Free PMC article. Review. - Expression Analysis of α5 Integrin Subunit Reveals Its Upregulation as a Negative Prognostic Biomarker for Glioblastoma.
Etienne-Selloum N, Prades J, Bello-Roufai D, Boone M, Sevestre H, Trudel S, Caillet P, Coutte A, Desenclos C, Constans JM, Martin S, Choulier L, Chauffert B, Dontenwill M. Etienne-Selloum N, et al. Pharmaceuticals (Basel). 2021 Aug 30;14(9):882. doi: 10.3390/ph14090882. Pharmaceuticals (Basel). 2021. PMID: 34577582 Free PMC article. - The role of caveolin-1 in tumors of the brain - functional and clinical implications.
Eser Ocak P, Ocak U, Tang J, Zhang JH. Eser Ocak P, et al. Cell Oncol (Dordr). 2019 Aug;42(4):423-447. doi: 10.1007/s13402-019-00447-x. Epub 2019 Apr 16. Cell Oncol (Dordr). 2019. PMID: 30993541 Review. - Vasohibin-2 modulates tumor onset in the gastrointestinal tract by normalizing tumor angiogenesis.
Kitahara S, Suzuki Y, Morishima M, Yoshii A, Kikuta S, Shimizu K, Morikawa S, Sato Y, Ezaki T. Kitahara S, et al. Mol Cancer. 2014 May 4;13:99. doi: 10.1186/1476-4598-13-99. Mol Cancer. 2014. PMID: 24885408 Free PMC article. - Shifting perspectives from "oncogenic" to oncofetal proteins; how these factors drive placental development.
West RC, Bouma GJ, Winger QA. West RC, et al. Reprod Biol Endocrinol. 2018 Oct 19;16(1):101. doi: 10.1186/s12958-018-0421-3. Reprod Biol Endocrinol. 2018. PMID: 30340501 Free PMC article. Review.
References
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. - PubMed
- Yuan F, Chen Y, Dellian M, Safabakhsh N, Ferrara N, Jain RK. Time-dependent vascular regression and permeability changes in established human tumor xenografts induced by an anti-vascular endothelial growth factor/vascular permeability factor antibody. Proc Natl Acad Sci USA. 1996;93:14765–14770. - PMC - PubMed
- Shaheen RM, Davis DW, Liu W, Zebrowski BK, Wilson MR, Bucana CD, McConkey DJ, McMahon G, Ellis LM. Antiangiogenic therapy targeting the tyrosine kinase receptor for vascular endothelial growth factor receptor inhibits the growth of colon cancer liver metastasis and induces tumor and endothelial cell apoptosis. Cancer Res. 1999;59:5412–5416. - PubMed
- Huang J, Frischer JS, Serur A, Kadenhe A, Yokoi A, McCrudden KW, New T, O’Toole K, Zabski S, Rudge JS, Holash J, Yancopoulos GD, Yamashiro DJ, Kandel JJ. Regression of established tumors and metastases by potent vascular endothelial growth factor blockade. Proc Natl Acad Sci USA. 2003;100:7785–7790. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL-24136/HL/NHLBI NIH HHS/United States
- P01 HL024136/HL/NHLBI NIH HHS/United States
- P50-CA90270/CA/NCI NIH HHS/United States
- P50 CA090270/CA/NCI NIH HHS/United States
- R01 HL059157/HL/NHLBI NIH HHS/United States
- HL-59157/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous