Hypophosphatemia leads to rickets by impairing caspase-mediated apoptosis of hypertrophic chondrocytes - PubMed (original) (raw)
Comparative Study
. 2005 Jul 5;102(27):9637-42.
doi: 10.1073/pnas.0502249102. Epub 2005 Jun 23.
Affiliations
- PMID: 15976027
- PMCID: PMC1172249
- DOI: 10.1073/pnas.0502249102
Comparative Study
Hypophosphatemia leads to rickets by impairing caspase-mediated apoptosis of hypertrophic chondrocytes
Yves Sabbagh et al. Proc Natl Acad Sci U S A. 2005.
Abstract
Rickets is seen in association with vitamin D deficiency and in several genetic disorders associated with abnormal mineral ion homeostasis. Studies in vitamin D receptor (VDR)-null mice have demonstrated that expansion of the late hypertrophic chondrocyte layer, characteristic of rickets, is secondary to impaired apoptosis of these cells. The observation that normalization of mineral ion homeostasis in the VDR-null mice prevents rachitic changes suggests that rickets is secondary to hypocalcemia, hypophosphatemia, or hyperparathyroidism, rather than impaired VDR action. To determine which of these abnormalities is responsible for impaired chondrocyte apoptosis and subsequent rachitic changes, two additional models were examined: diet-induced hypophosphatemia/hypercalcemia and hypophosphatemia secondary to mutations in the Phex gene. The former model is associated with suppressed parathyroid hormone levels as a consequence of hypercalcemia. The latter model demonstrates normal calcium and parathyroid hormone levels, but 1,25-dihydroxyvitamin D levels that are inappropriately low for the degree of hypophosphatemia. Our studies demonstrate that normal phosphorus levels are required for growth plate maturation and implicate a critical role for phosphate-regulated apoptosis of hypertrophic chondrocytes via activation of the caspase-9-mediated mitochondrial pathway.
Figures
Fig. 1.
Histological analysis and evaluation of hypertrophic chondrocyte apoptosis in 24-day-old mice. Shown are hematoxylin/eosin-stained sections (A and C) and TUNEL assay (B and D). (A and B) Wild-type (WT) and VDR knockout (KO) mice fed a regular diet (Left) or a rescue diet (Right) that normalizes mineral ion homeostasis. (C and D) Wild-type mice fed a regular diet, Hyp mice fed a regular diet, and wild-type mice fed a high-calcium/low-phosphate diet from 18 to 24 days of age. Brackets indicate the hypertrophic chondrocyte layer. Data are representative of experiments performed on sections from three or more mice for each condition. EV, Evans blue; T, TUNEL.
Fig. 2.
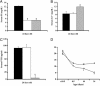
Biochemical parameters. Serum phosphate (A), serum calcium (B), and immunoreactive PTH (C) were measured in 24-day-old wild-type mice fed a regular diet (black bars), Hyp mice fed a regular diet (white bars), and wild-type mice fed a high-calcium/low-phosphate diet (gray bars). (D) Serum phosphate levels at 18.5 days postcoitum and at 0.5, 10, and 24 days of age in wild-type (♦) and Hyp (○) mice fed a regular diet. Data represent the mean ± SD of values from five mice for each assay. *, P ≤ 0.05.
Fig. 3.
Histological analysis and evaluation of hypertrophic chondrocyte apoptosis in Hyp mice. Hematoxylin/eosin staining (A and C) and TUNEL assays (B and D) were performed on sections from wild-type (WT) and Hyp mice at 0.5 day (A and B) and 10 days of age (C and D). Brackets indicate the hypertrophic chondrocyte layer. Data are representative of experiments performed on sections from three or more mice for each condition. EV, Evans blue; T, TUNEL.
Fig. 4.
Growth-plate mineralization in 24-day-old mice. von Kossa staining was performed on sections from 24-day-old wild-type mice fed a regular diet, Hyp mice fed a regular diet, and wild-type mice fed a high-calcium/low-phosphate diet from 18 to 24 days of age. Insets show a magnification of the hypertrophic chondrocytes. Brackets indicate the hypertrophic chondrocyte layer. Data are representative of sections from five mice for each condition.
Fig. 5.
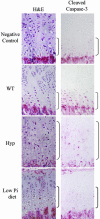
Cleaved caspase-3 immunohistochemistry. Sections from 24-day-old wild-type (WT) mice fed a regular diet, Hyp mice fed a regular diet, and mice fed a high-calcium/low-phosphate diet from 18 to 24 days of age were immunostained with antibody to cleaved caspase-3. Brackets indicate the hypertrophic chondrocyte layer. Data are representative of experiments performed on sections from three or more mice for each condition.
Fig. 6.
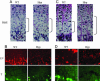
Phosphate induces hypertrophic chondrocyte apoptosis in vitro. Hypertrophic chondrocytes were treated with 7 mM NaCl or 7 mM NaH2PO4 for 18 h in the presence or absence of 1 μM cyclosporin A (CsA). Cell lysates were subjected to Western blot analysis using anti-caspase-9. Lysates of 3T3 fibroblasts and proliferating chondrocytes (PC) treated with 7 mM NaH2PO4 were similarly analyzed. Data are representative of those obtained from three independent cell preparations.
Fig. 7.
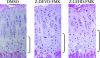
In vivo inhibition of caspase-3 or caspase-9 leads to expansion of the hypertrophic chondrocyte layer. Shown are hematoxylin/eosin-stained sections from 24-day-old mice injected at days 18–23 with DMSO, caspase-3 inhibitor (Z-DEVD-FMK), or caspase-9 inhibitor (Z-LEHD-FMK). Brackets indicate the hypertrophic chondrocyte layer.
Similar articles
- Impaired Growth Plate Maturation in XLH Is due to Both Excess FGF23 and Decreased 1,25-Dihydroxyvitamin D Signaling.
Yadav PS, Kobelski MM, Martins JS, Tao T, Liu ES, Demay MB. Yadav PS, et al. Endocrinology. 2023 Nov 20;165(1):bqad186. doi: 10.1210/endocr/bqad186. Endocrinology. 2023. PMID: 38066669 - Rickets in VDR null mice is secondary to decreased apoptosis of hypertrophic chondrocytes.
Donohue MM, Demay MB. Donohue MM, et al. Endocrinology. 2002 Sep;143(9):3691-4. doi: 10.1210/en.2002-220454. Endocrinology. 2002. PMID: 12193585 - The receptor-dependent actions of 1,25-dihydroxyvitamin D are required for normal growth plate maturation in NPt2a knockout mice.
Miedlich SU, Zhu ED, Sabbagh Y, Demay MB. Miedlich SU, et al. Endocrinology. 2010 Oct;151(10):4607-12. doi: 10.1210/en.2010-0354. Epub 2010 Aug 4. Endocrinology. 2010. PMID: 20685875 Free PMC article. - Mechanism of vitamin D receptor action.
Demay MB. Demay MB. Ann N Y Acad Sci. 2006 Apr;1068:204-13. doi: 10.1196/annals.1346.026. Ann N Y Acad Sci. 2006. PMID: 16831920 Review. - Hypophosphatemia: the common denominator of all rickets.
Tiosano D, Hochberg Z. Tiosano D, et al. J Bone Miner Metab. 2009;27(4):392-401. doi: 10.1007/s00774-009-0079-1. Epub 2009 Jun 6. J Bone Miner Metab. 2009. PMID: 19504043 Review.
Cited by
- Debilitating Musculoskeletal Disease in Two Free-Ranging Juvenile American Black Bears (Ursus americanus).
Fahrenholz IC, Dennis MM, Morandi F, Dittmer KE, Sheldon JD. Fahrenholz IC, et al. Animals (Basel). 2024 Jul 17;14(14):2088. doi: 10.3390/ani14142088. Animals (Basel). 2024. PMID: 39061550 Free PMC article. - NFATc1 Is Required for Vitamin D- and Phosphate-Mediated Regulation of Osteocyte Lacuno-Canalicular Remodeling.
Jagga S, Hughes A, Manoochehri Arash N, Sorsby M, Brooks DJ, Divieti Pajevic P, Liu ES. Jagga S, et al. Endocrinology. 2024 Jul 1;165(8):bqae087. doi: 10.1210/endocr/bqae087. Endocrinology. 2024. PMID: 39024412 - Lessons learned from the real-world diagnosis and management of hereditary hypophosphatemic rickets.
Chaturvedi D, Mehasi TE, Benbrahim A, ElDeeb L, Deeb A. Chaturvedi D, et al. Bone Rep. 2024 Mar 21;21:101753. doi: 10.1016/j.bonr.2024.101753. eCollection 2024 Jun. Bone Rep. 2024. PMID: 39011543 Free PMC article. - Relationships between matrix mineralization, oxidative metabolism, and mitochondrial structure during ATDC5 murine chondroprogenitor cell line differentiation.
Blank K, Ekanayake D, Cooke M, Bragdon B, Hussein A, Gerstenfeld L. Blank K, et al. J Cell Physiol. 2024 Aug;239(8):e31285. doi: 10.1002/jcp.31285. Epub 2024 Jun 11. J Cell Physiol. 2024. PMID: 38860464 - Impaired Growth Plate Maturation in XLH Is due to Both Excess FGF23 and Decreased 1,25-Dihydroxyvitamin D Signaling.
Yadav PS, Kobelski MM, Martins JS, Tao T, Liu ES, Demay MB. Yadav PS, et al. Endocrinology. 2023 Nov 20;165(1):bqad186. doi: 10.1210/endocr/bqad186. Endocrinology. 2023. PMID: 38066669
References
- St.-Arnaud, R., Messerlian, S., Moir, J. M., Omdahl, J. L. & Glorieux, F. H. (1997) J. Bone Miner. Res. 12, 1552-1559. - PubMed
- Hughes, M. R., Malloy, P. J., Kieback, D. G., Kesterson, R. A., Pike, J. W., Feldman, D. & O'Malley, B. W. (1988) Science 242, 1702-1705. - PubMed
- Sabbagh, Y., Jones, A. O. & Tenenhouse, H. S. (2000) Hum. Mutat. 16, 1-6. - PubMed
- The Autosomal Dominant Hypophosphataemic Rickets Consortium (2000) Nat. Genet. 26, 345-348. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases