Mammary stem cells, self-renewal pathways, and carcinogenesis - PubMed (original) (raw)
Review
Mammary stem cells, self-renewal pathways, and carcinogenesis
Suling Liu et al. Breast Cancer Res. 2005.
Abstract
The mammary gland epithelial components are thought to arise from stem cells that undergo both self-renewal and differentiation. Self-renewal has been shown to be regulated by the Hedgehog, Notch, and Wnt pathways and the transcription factor B lymphoma Mo-MLV insertion region 1 (Bmi-1). We review data about the existence of stem cells in the mammary gland and the pathways regulating the self-renewal of these cells. We present evidence that deregulation of the self-renewal in stem cells/progenitors might be a key event in mammary carcinogenesis. If 'tumor stem cells' are inherently resistant to current therapies, targeting stem cell self-renewal pathways might provide a novel approach for breast cancer treatment.
Figures
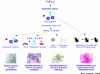
Figure 1
Experimental design for assessing self-renewal and differentiation potential of cells grown as mammospheres. (a) Self-renewal is assessed by evaluating the ability of mammosphere-derived cells to form new spheres, containing multipotent cells. (b) Differentiation into all the three mammary lineage types on collagen in the presence of serum [immunostained with lineage-specific markers: brown, ductal epithelial (ESA); purple, myoepithelial (CD10); red, alveolar (β-casein)]. (c) Generate complex ductal-alveolar structures in three-dimensional Matrigel culture. (d) Differentiation and self-renewal in vivo are tested by implanting human mammary epithelial cells into the cleared mammary fat pads of immunodeficient mice (NOD/SCID mice). EGF, epidermal growth factor.
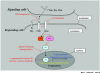
Figure 2
A schematic diagram for the hedgehog (HH) signaling pathway. Ligands, such as Sonic Hedgehog (Shh), Indian Hedgehog (Ihh), and Desert Hedgehog (Dhh), are secreted by signaling cells and bind the transmembrane receptor patched (Ptch) in HH responding cells. In the absence of ligands, Ptch binds to Smoothened (Smo) and blocks Smo's function, whereas this inhibition is relieved in the presence of ligands, and Smo initiates a signaling cascade that results in the release of transcription factors Glis from cytoplasmic proteins fused (Fu) and suppressor of fused (SuFu). In the inactive situation, SuFu prevents Glis from translocating to the nucleus; in the active situation, Fu inhibits SuFu and Glis are released. Gli proteins translocate into the nucleus and control target gene transcription. The red lines and the agents in red show the inhibitors of this pathway with potential therapeutic value.
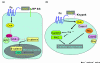
Figure 3
A schematic diagram for the Notch signaling pathway. Upon binding of the DSL ligand, Notch signaling is modulated by fringe, and Notch receptors are activated by serial cleavage events involving members of the ADAM (for 'a disintegrin and metalloproteinase') protease family, as well as an intramembrane cleavage regulated by γ-secretase (presenilin). This intramembrane cleavage is followed by translocation of the intracellular domain on Notch to the nucleus, where it acts on downstream targets. CBF, C promoter binding factor; HDAC, histone deacetylase; HAT, histone acetyltransferase.
Figure 4
A schematic diagram for the Wnt signaling pathway. (a) The canonical Wnt/β-catenin pathway. Canonical Wnt signaling requires the Frizzled (Fz) and low-density lipoprotein receptor-related proteins 5 and 6 (LRP5/6) co-receptors to activate Dishevelled (Dsh). Then Dsh inhibits the activity of the β-catenin destruction complex (adenomatous polyposis coli (APC), axin, and glycogen synthase kinase-3 (GSK-3)), which phosphorylates β-catenin in the absence of the ligands. β-Catenin is stabilized and translocated to the nucleus, where it recruits transactivators to high mobility group (HMG)-box DNA-binding proteins of the lymphoid enhancer factor/T cell factor (LEF/TCF) family. (b) The noncanonical Wnt signaling pathway. Noncanonical Wnt signaling requires Frizzled receptors and the proteoglycan co-receptor Knypek. In this pathway, Dsh localizes to the cell membrane through its DEP domain. A main branch downstream of Dsh involves the small GTPases of the Rho family. Dsh activation of Rho requires the bridging molecule Daam1. Dsh can also stimulate calcium flux and the activation of the calcium-sensitive kinases protein kinase C (PKC) and calmodulin-dependent protein kinase II (CanKII). At the end, the activation of this pathway induces the complex and dynamic cellular response.
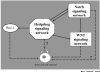
Figure 5
A hypothetic interacting model in the regulation of stem cell self-renewal by the Hedgehog signaling pathway, the Notch signaling pathway, the Wnt signaling pathway, and B lymphoma Mo-MLV insertion region 1 (Bmi-1). Interactions between the Hedgehog, Notch, and Wnt pathways and Bmi-1 are shown by solid arrows; interactions between stem cell self-renewal regulation by the pathways and Bmi-1 are shown by dashed arrows; the question marks represent the postulated interactions.
Similar articles
- On mammary stem cells.
Woodward WA, Chen MS, Behbod F, Rosen JM. Woodward WA, et al. J Cell Sci. 2005 Aug 15;118(Pt 16):3585-94. doi: 10.1242/jcs.02532. J Cell Sci. 2005. PMID: 16105882 Review. - Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells.
Liu S, Dontu G, Mantle ID, Patel S, Ahn NS, Jackson KW, Suri P, Wicha MS. Liu S, et al. Cancer Res. 2006 Jun 15;66(12):6063-71. doi: 10.1158/0008-5472.CAN-06-0054. Cancer Res. 2006. PMID: 16778178 Free PMC article. - Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells.
Dontu G, Jackson KW, McNicholas E, Kawamura MJ, Abdallah WM, Wicha MS. Dontu G, et al. Breast Cancer Res. 2004;6(6):R605-15. doi: 10.1186/bcr920. Epub 2004 Aug 16. Breast Cancer Res. 2004. PMID: 15535842 Free PMC article. - Mammary stem and progenitor cell regulation.
Glazer RI, Wang X, Yuan H, Yin Y. Glazer RI, et al. Cancer Biomark. 2007;3(4-5):171-81. doi: 10.3233/cbm-2007-34-502. Cancer Biomark. 2007. PMID: 17917147 Review. - Mammary development and breast cancer: the role of stem cells.
Ercan C, van Diest PJ, Vooijs M. Ercan C, et al. Curr Mol Med. 2011 Jun;11(4):270-85. doi: 10.2174/156652411795678007. Curr Mol Med. 2011. PMID: 21506923 Free PMC article. Review.
Cited by
- The role of maintenance proteins in the preservation of epithelial cell identity during mammary gland remodeling and breast cancer initiation.
Coradini D, Oriana S. Coradini D, et al. Chin J Cancer. 2014 Feb;33(2):51-67. doi: 10.5732/cjc.013.10040. Epub 2013 Jul 12. Chin J Cancer. 2014. PMID: 23845141 Free PMC article. Review. - The Patentability of Stem Cells in Australia.
Petering J, Cowin P. Petering J, et al. Cold Spring Harb Perspect Med. 2015 Jul 1;5(10):a020966. doi: 10.1101/cshperspect.a020966. Cold Spring Harb Perspect Med. 2015. PMID: 26134481 Free PMC article. Review. - CXCL12γ induces human prostate and mammary gland development.
Jung Y, Kim JK, Lee E, Cackowski FC, Decker AM, Krebsbach PH, Taichman RS. Jung Y, et al. Prostate. 2020 Sep;80(13):1145-1156. doi: 10.1002/pros.24043. Epub 2020 Jul 13. Prostate. 2020. PMID: 32659025 Free PMC article. - Activation of Smad-mediated TGF-β signaling triggers epithelial-mesenchymal transitions in murine cloned corneal progenitor cells.
Kawakita T, Espana EM, Higa K, Kato N, Li W, Tseng SC. Kawakita T, et al. J Cell Physiol. 2013 Jan;228(1):225-34. doi: 10.1002/jcp.24126. J Cell Physiol. 2013. PMID: 22674610 Free PMC article. - The Role of Intra-Tumoral Heterogeneity and Its Clinical Relevance in Epithelial Ovarian Cancer Recurrence and Metastasis.
Roberts CM, Cardenas C, Tedja R. Roberts CM, et al. Cancers (Basel). 2019 Jul 30;11(8):1083. doi: 10.3390/cancers11081083. Cancers (Basel). 2019. PMID: 31366178 Free PMC article. Review.
References
- Rudland PS, Barraclough R, Fernig DG, Smith JA. Mammary stem cells in normal development and cancer. In: Potten CS, editor. Stem Cells. San Diego: Academic Press; 1997. pp. 147–232.
- Stingl J, Eaves CJ, Kuusk U, Emerman JT. Phenotypic and functional characterization in vitro of a multipotent epithelial cell present in the normal adult human breast. Differentiation. 1998;63:201–213. - PubMed
- Karsten U, Papsdorf G, Pauly A, Vojtesek B, Moll R, Lane EB, Clausen H, Stosiek P, Kasper M. Subtypes of non-transformed human mammary epithelial cells cultured in vitro: histo-blood group antigen H type 2 defines basal cell-derived cells. Differentiation. 1993;54:55–66. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical