Mice with genetically altered glucocorticoid receptor expression show altered sensitivity for stress-induced depressive reactions - PubMed (original) (raw)
. 2005 Jun 29;25(26):6243-50.
doi: 10.1523/JNEUROSCI.0736-05.2005.
Sabine Chourbaji, Rainer Hellweg, Alexandre Urani, Christiane Zacher, Wolfgang Schmid, Mathias Zink, Heide Hörtnagl, Herta Flor, Fritz A Henn, Günther Schütz, Peter Gass
Affiliations
- PMID: 15987954
- PMCID: PMC6725059
- DOI: 10.1523/JNEUROSCI.0736-05.2005
Mice with genetically altered glucocorticoid receptor expression show altered sensitivity for stress-induced depressive reactions
Stephanie Ridder et al. J Neurosci. 2005.
Abstract
Altered glucocorticoid receptor (GR) signaling is a postulated mechanism for the pathogenesis of major depression. To mimic the human situation of altered GR function claimed for depression, we generated mouse strains that underexpress or overexpress GR, but maintain the regulatory genetic context controlling the GR gene. To achieve this goal, we used the following: (1) GR-heterozygous mutant mice (GR+/-) with a 50% GR gene dose reduction, and (2) mice overexpressing GR by a yeast artificial chromosome resulting in a twofold gene dose elevation. GR+/- mice exhibit normal baseline behaviors but demonstrate increased helplessness after stress exposure, a behavioral correlate of depression in mice. Similar to depressed patients, GR+/- mice have a disinhibited hypothalamic-pituitary-adrenal (HPA) system and a pathological dexamethasone/corticotropin-releasing hormone test. Thus, they represent a murine depression model with good face and construct validity. Overexpression of GR in mice evokes reduced helplessness after stress exposure, and an enhanced HPA system feedback regulation. Therefore, they may represent a model for a stress-resistant strain. These mouse models can now be used to study biological changes underlying the pathogenesis of depressive disorders. As a first potential molecular correlate for such changes, we identified a downregulation of BDNF protein content in the hippocampus of GR+/- mice, which is in agreement with the so-called neurotrophin hypothesis of depression.
Figures
Figure 1.
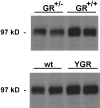
Expression levels of GR protein in GR+/- and YGR mice in the hippocampus. Western blot analyses show a marked downregulation of GR in GR+/- mice compared with wild-type littermates (top gel; each lane represents a hippocampal protein extract from a different animal). Conversely, YGR mice exhibit a distinct GR upregulation compared with their control littermates (bottom gel). wt, Wild type.
Figure 2.
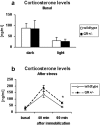
Corticosterone levels in GR+/- mice under basal and stressful conditions. a, Plasma corticosterone levels are unchanged in GR+/- mice compared with wild-type mice, both during the animals' active (dark) and inactive (light) phase. b, After stress exposure, however, corticosterone levels are higher in GR+/- mice at 40 and 60 min (*p < 0.05) after immobilization.
Figure 3.
GR+/- mice display increased helplessness in a shuttle box test after exposure to inescapable footshocks on the 2 d preceding the test. a, GR+/- mice exhibit significantly increased escape latencies compared with their wild-type littermates (*p < 0.05). b, GR+/- mice also show a higher number of escape failures (*p < 0.05). c, Latencies and numbers of failures are highly correlated when plotted for individual mutant and wild-type mice, demonstrating that the subjects with the worst coping (in the top right area of the graph) are all GR+/- mice.
Figure 4.
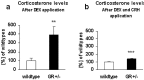
GR+/- mice are nonsuppressors in the Dex/CRH test. a, After administration of dexamethasone, wild-type mice show decreased corticosterone levels, whereas GR+/- mice exhibit nonsuppression (**p < 0.01). b, In the combined Dex/CRH test, corticosterone levels are significantly elevated in GR+/- mice compared with the levels of wild-type mice (***p < 0.001).
Figure 5.
YGR mice have a stress-resistant HPA system and are oversuppressors in the Dex test. a, After stress exposure, corticosterone levels are significantly lower in YGR mice at 40 min after immobilization than in wild-type controls (**p < 0.05). b, After administration of dexamethasone, YGR mice show a significant oversuppression of corticosterone levels compared with wild-type mice (**p < 0.01). c, In the combined Dex/CRH test, corticosterone levels are lower in YGR mice compared with the levels of wild-type mice (p = 0.07).
Figure 6.
YGR mice are more resistant in the learned-helplessness paradigm. a, YGR mice exhibit significantly decreased escape failures compared with their wild-type littermates (*p < 0.05). b, YGR mice also show a reduced number of escape latencies (*p<0.05). c, Latencies and numbers of failures are highly correlated when plotted for individual mutant and wild-type mice, demonstrating worse coping (in the top right area of the graph) in wild-type mice, whereas YGR mice cluster in the bottom left area of the graph.
Figure 7.
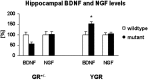
BDNF protein levels in the hippocampus are significantly diminished in GR+/- mice (p < 0.05) and significantly increased in YGR mice (*p < 0.05). In contrast, the expression levels of NGF are unaltered in both mutant strains.
Similar articles
- May the use of different background strains 'strain' the stress-related phenotype of GR+/- mice?
Vogt MA, Pfeiffer N, Le Guisquet AM, Brandwein C, Brizard B, Gass P, Belzung C, Chourbaji S. Vogt MA, et al. Behav Brain Res. 2017 Sep 29;335:71-79. doi: 10.1016/j.bbr.2017.07.037. Epub 2017 Aug 3. Behav Brain Res. 2017. PMID: 28782590 - Depression-prone mice with reduced glucocorticoid receptor expression display an altered stress-dependent regulation of brain-derived neurotrophic factor and activity-regulated cytoskeleton-associated protein.
Molteni R, Calabrese F, Chourbaji S, Brandwein C, Racagni G, Gass P, Riva MA. Molteni R, et al. J Psychopharmacol. 2010 Apr;24(4):595-603. doi: 10.1177/0269881108099815. Epub 2008 Dec 12. J Psychopharmacol. 2010. PMID: 19074532 - Mice that under- or overexpress glucocorticoid receptors as models for depression or posttraumatic stress disorder.
Chourbaji S, Vogt MA, Gass P. Chourbaji S, et al. Prog Brain Res. 2008;167:65-77. doi: 10.1016/S0079-6123(07)67005-8. Prog Brain Res. 2008. PMID: 18037007 Review. - Metabotropic glutamate receptor subtype 7 ablation causes dysregulation of the HPA axis and increases hippocampal BDNF protein levels: implications for stress-related psychiatric disorders.
Mitsukawa K, Mombereau C, Lötscher E, Uzunov DP, van der Putten H, Flor PJ, Cryan JF. Mitsukawa K, et al. Neuropsychopharmacology. 2006 Jun;31(6):1112-22. doi: 10.1038/sj.npp.1300926. Neuropsychopharmacology. 2006. PMID: 16237391 - How to measure glucocorticoid receptor's sensitivity in patients with stress-related psychiatric disorders.
Leistner C, Menke A. Leistner C, et al. Psychoneuroendocrinology. 2018 May;91:235-260. doi: 10.1016/j.psyneuen.2018.01.023. Epub 2018 Feb 2. Psychoneuroendocrinology. 2018. PMID: 29449045 Review.
Cited by
- Mineralocorticoid receptors dampen glucocorticoid receptor sensitivity to stress via regulation of FKBP5.
Hartmann J, Bajaj T, Klengel C, Chatzinakos C, Ebert T, Dedic N, McCullough KM, Lardenoije R, Joëls M, Meijer OC, McCann KE, Dudek SM, Sarabdjitsingh RA, Daskalakis NP, Klengel T, Gassen NC, Schmidt MV, Ressler KJ. Hartmann J, et al. Cell Rep. 2021 Jun 1;35(9):109185. doi: 10.1016/j.celrep.2021.109185. Cell Rep. 2021. PMID: 34077736 Free PMC article. - Blunted hypothalamo-pituitary adrenal axis response to predator odor predicts high stress reactivity.
Whitaker AM, Gilpin NW. Whitaker AM, et al. Physiol Behav. 2015 Aug 1;147:16-22. doi: 10.1016/j.physbeh.2015.03.033. Epub 2015 Mar 27. Physiol Behav. 2015. PMID: 25824191 Free PMC article. - Behavioral Animal Models and Neural-Circuit Framework of Depressive Disorder.
Tian X, Russo SJ, Li L. Tian X, et al. Neurosci Bull. 2024 Aug 9. doi: 10.1007/s12264-024-01270-7. Online ahead of print. Neurosci Bull. 2024. PMID: 39120643 Review. - Convergence of endothelial dysfunction, inflammation and glucocorticoid resistance in depression-related cardiovascular diseases.
Hage Z, Madeira MM, Koliatsis D, Tsirka SE. Hage Z, et al. BMC Immunol. 2024 Sep 27;25(1):61. doi: 10.1186/s12865-024-00653-9. BMC Immunol. 2024. PMID: 39333855 Free PMC article. Review. - Early response to venlafaxine antidepressant correlates with lower ACTH levels prior to pharmacological treatment.
Araya AV, Rojas P, Fritsch R, Rojas R, Herrera L, Rojas G, Gatica H, Silva H, Fiedler JL. Araya AV, et al. Endocrine. 2006 Dec;30(3):289-98. doi: 10.1007/s12020-006-0007-2. Endocrine. 2006. PMID: 17526941 Clinical Trial.
References
- Altar CA (1999) Neurotrophins and depression. Trends Pharmacol Sci 20: 59-61. - PubMed
- Banbury Conference (1997) Mutant mice and neuroscience: recommendations concerning genetic background. Banbury Conference on genetic background in mice. Neuron 19: 755-759. - PubMed
- Belanoff JK, Rothschild AJ, Cassidy F, DeBattista C, Baulieu EE, Schold C, Schatzberg AF (2002) An open label trial of C-1073 (mifepristone) for psychotic major depression. Biol Psychiatry 52: 386-392. - PubMed
- Chao HM, Sakai RR, Ma LY, McEwen BS (1998) Adrenal steroid regulation of neurotrophic factor expression in the rat hippocampus. Endocrinology 139: 3112-3118. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials