Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses - PubMed (original) (raw)
Review
Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses
Christine H Foyer et al. Plant Cell. 2005 Jul.
No abstract available
Figures
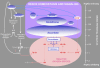
Figure 1.
Reductant–Antioxidant–Oxidant Interactions in Redox Homeostasis and Signaling. Nonenzymic components are positioned on a nonlinear scale (right) at their approximate electrochemical potential in volts. In nonquiescent cells under optimal conditions, large pools of glutathione and ascorbate are maintained in a highly reduced state, and buffer ROS that are continuously produced by oxidases or by electron transport components, such as FeS centers, semiquinones, or (as depicted) ferredoxin. Other key redox signaling components are thioredoxins (TRX) and glutaredoxins (GRX), which are reduced by ferredoxin, NADPH, or glutathione. Production of the superoxide anion (OO.−) and H2O2 (HOOH) can be induced or promoted under certain conditions, leading to increased oxidative charge on the reductant-antioxidant system. Reductive cleavage of H2O2 produces the hydroxyl radical (OH·), an extremely reactive electron and hydrogen acceptor whose reduction potentially involves indiscriminate oxidation of cellular components. Excessive production of OH· is avoided by enzymatic processing of H2O2 to water by peroxidases or to water and O2 by catalases. Signaling linked to increased availability of ROS may be caused, limited, or mediated by changes in antioxidant capacity (see text).
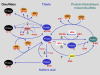
Figure 2.
Sensitive Control of Cellular Redox Responses by Protein Thiol Status. The numbered reactions are as follows. 1, Reversible dithiol-disulfide formation mediated by the thioredoxin (or glutaredoxin) system, as in light modulation of chloroplastic enzyme activities. 2, Direct H2O2-dependent oxidation of dithiol sensor proteins, such as the bacterial oxyR, reversed by GRX. 3, Peroxidase-catalyzed H2O2 sensing, as occurs with the yeast yAP-1 protein, which can be reversed by TRX. 4, Sulfenic acid formation, as occurs in the bacterial transcription factor OhrR or in peroxiredoxins. The sulfenic acid can be directly reduced by glutathione or lipoic acid (in 1-Cys peroxiredoxins) or give rise to an intramolecular or intermolecular disulfide bond that is reducible to thiols by thioredoxins or glutaredoxins in 2-Cys peroxiredoxins. 5, Glutathionylation through thiol-disulfide exchange of protein thiols with GSSG. 6, ROS-catalyzed glutathionylation via thiyl radical formation. 7, Glutaredoxin-catalyzed glutathionylation reaction. Whatever the route of glutathionylation, the process may be reversed by certain glutaredoxins and/or thioredoxins. Only some of the possible routes for changes in Cys sulfur status are shown. Other modifications, such as protein thiol nitrosylation, could also be important, as could more oxidized forms of protein Cys sulfur (sulfinic and sulfonic acids) and glutathionylation via nitrosylated glutathione. GRX, glutaredoxin; GSH, glutathione; GSSG, glutathione disulfide; POX, peroxidase; TRX, thioredoxin; XRX, thioredoxin or glutaredoxin.
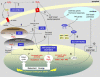
Figure 3.
Oxidant and Antioxidant Signaling in Cell Death and Acclimation Responses: Major Components of the Metabolic Interface between Plant Stresses and Orchestrated Responses. The hypothetical scheme draws together some of the redox-modulated elements recently identified in plants and others likely to be influential. Components that promote an oxidative signal are shown in red, and those that oppose such signals or transmit a reductive signal are shown in dark blue. Plasma membrane NADPH oxidases are activated by elicitor or hormone-mediated signaling and produce H2O2 (1). Production of H2O2 can oxidize putative membrane receptors, and this function is opposed by apoplastic ascorbate (2). ROS production activates signaling through specific MAP kinases (3), and some of these components are also important in ROS-mediated hormone and growth responses. A key factor in specification of pathogenesis and cell death responses is secondary production of ROS, either by positive feedback enhancement of the primary oxidative signal (4) or by withdrawal and inactivation of antioxidative capacity. Downregulation of the antioxidative system could occur by posttranslational modulation of heme functions by salicylic acid and nitric oxide (5) and/or programmed withdrawal of antioxidative enzyme expression (6). Oxidative inhibition of chloroplast metabolism by glutathionylation and/or inactivation of thioredoxin-modulated enzymes may also be crucial in increasing chloroplastic flux to oxygen to enhance ROS production (7). Greater availability of ROS activates glutathione synthesis (8), and the resulting increase in cytosolic glutathione is somehow linked to induction or activation of a thioredoxin or glutaredoxin that is able to reduce NPR1 (9). NPR1 reduction is associated with its accumulation in the nucleus and its interaction with TGA transcription factors to induce PR gene expression. Enhanced chloroplastic levels of ROS such as singlet oxygen may also activate the pathways that (10) set in train the cell death program. For discussion and references, see text. APX, ascorbate peroxidase; CAT, catalase; CIV, cytochrome oxidase; SOD, superoxide dismutase.
Similar articles
- Stress homeostasis - the redox and auxin perspective.
Tognetti VB, Mühlenbock P, Van Breusegem F. Tognetti VB, et al. Plant Cell Environ. 2012 Feb;35(2):321-33. doi: 10.1111/j.1365-3040.2011.02324.x. Epub 2011 Apr 26. Plant Cell Environ. 2012. PMID: 21443606 Review. - Higher plant antioxidants and redox signaling under environmental stresses.
Shao HB, Chu LY, Shao MA, Jaleel CA, Mi HM. Shao HB, et al. C R Biol. 2008 Jun;331(6):433-41. doi: 10.1016/j.crvi.2008.03.011. Epub 2008 Apr 28. C R Biol. 2008. PMID: 18510996 Review. - Signal transduction during oxidative stress.
Vranová E, Inzé D, Van Breusegem F. Vranová E, et al. J Exp Bot. 2002 May;53(372):1227-36. J Exp Bot. 2002. PMID: 11997371 Review. - Cell signaling and receptors with resorcinols and flavonoids: redox, reactive oxygen species, and physiological effects.
Kovacic P, Somanathan R. Kovacic P, et al. J Recept Signal Transduct Res. 2011 Aug;31(4):265-70. doi: 10.3109/10799893.2011.586353. J Recept Signal Transduct Res. 2011. PMID: 21745156 Review. - Cellular redox regulation, signaling, and stress response in plants.
Shigeoka S, Maruta T. Shigeoka S, et al. Biosci Biotechnol Biochem. 2014;78(9):1457-70. doi: 10.1080/09168451.2014.942254. Biosci Biotechnol Biochem. 2014. PMID: 25209493 Review.
Cited by
- The influence of metal stress on the availability and redox state of ascorbate, and possible interference with its cellular functions.
Bielen A, Remans T, Vangronsveld J, Cuypers A. Bielen A, et al. Int J Mol Sci. 2013 Mar 20;14(3):6382-413. doi: 10.3390/ijms14036382. Int J Mol Sci. 2013. PMID: 23519107 Free PMC article. - Chloroplast-derived photo-oxidative stress causes changes in H2O2 and EGSH in other subcellular compartments.
Ugalde JM, Fuchs P, Nietzel T, Cutolo EA, Homagk M, Vothknecht UC, Holuigue L, Schwarzländer M, Müller-Schüssele SJ, Meyer AJ. Ugalde JM, et al. Plant Physiol. 2021 May 27;186(1):125-141. doi: 10.1093/plphys/kiaa095. Plant Physiol. 2021. PMID: 33793922 Free PMC article. - Senescence, Stress, and Reactive Oxygen Species.
Jajic I, Sarna T, Strzalka K. Jajic I, et al. Plants (Basel). 2015 Jul 8;4(3):393-411. doi: 10.3390/plants4030393. Plants (Basel). 2015. PMID: 27135335 Free PMC article. Review. - When Bad Guys Become Good Ones: The Key Role of Reactive Oxygen Species and Nitric Oxide in the Plant Responses to Abiotic Stress.
Farnese FS, Menezes-Silva PE, Gusman GS, Oliveira JA. Farnese FS, et al. Front Plant Sci. 2016 Apr 12;7:471. doi: 10.3389/fpls.2016.00471. eCollection 2016. Front Plant Sci. 2016. PMID: 27148300 Free PMC article. Review. - Transformation of plum plants with a cytosolic ascorbate peroxidase transgene leads to enhanced water stress tolerance.
Diaz-Vivancos P, Faize L, Nicolás E, Clemente-Moreno MJ, Bru-Martinez R, Burgos L, Hernández JA. Diaz-Vivancos P, et al. Ann Bot. 2016 Jun;117(7):1121-31. doi: 10.1093/aob/mcw045. Epub 2016 Apr 8. Ann Bot. 2016. PMID: 27059431 Free PMC article.
References
- Ameisen, J.C. (2002). On the origin, evolution, and nature of programmed cell death: A timeline of four billion years. Cell Death Differ. 9, 367–393. - PubMed
- Asada, K., and Takahashi, M. (1987). Production and scavenging of active oxygen species in photosynthesis. In Photoinhibition, D. Kyle, C. Osmond, and C. Arntzen, eds (New York: Elsevier Science Publishers), pp. 227–287.
- Ball, L., Accotto, G., Bechtold, U., Creissen, G., Funck, D., Jimenez, A., Kular, B., Leyland, N., Mejia-Carranza, J., Reynolds, H., Karpinski, S., and Mullineaux, P.M. (2004). Evidence for a direct link between glutathione biosynthesis and stress defense gene expression in Arabidopsis. Plant Cell 16, 2448–2462. - PMC - PubMed
- Balmer, Y., Vensel, W.H., Tanaka, C.K., Hurkman, W.J., Gelhaye, E., Rouhier, N., Jacquot, J.P., Manieri, W., Schürmann, P., Droux, M., and Buchanan, B. (2004). Thioredoxin links redox to the regulation of fundamental processes of plant mitochondria. Proc. Natl. Acad. Sci. USA 101, 2642–2647. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical