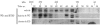Phosphorylation and stabilization of HURP by Aurora-A: implication of HURP as a transforming target of Aurora-A - PubMed (original) (raw)
Phosphorylation and stabilization of HURP by Aurora-A: implication of HURP as a transforming target of Aurora-A
Chang-Tze Ricky Yu et al. Mol Cell Biol. 2005 Jul.
Abstract
Aurora-A, a mitotic serine/threonine kinase with oncogene characteristics, has recently drawn intense attention because of its association with the development of human cancers and its relationship with mitotic progression. Using the gene expression profiles of Aurora-A as a template to search for and compare transcriptome expression profiles in publicly accessible microarray data sets, we identified HURP (encodes hepatoma upregulated protein) as one of the best Aurora-A-correlated genes. Empirical validation indicates that HURP has several characteristics in common with Aurora-A. These two genes have similar expression patterns in hepatocellular carcinoma, liver regeneration after partial hepatectomy, and cell cycle progression and across a variety of tissues and cell lines. Moreover, Aurora-A phosphorylated HURP in vitro and in vivo. Ectopic expression of either the catalytically inactive form of Aurora-A or the HURP-4P mutant, in which the Aurora-A phosphorylation sites were replaced with Ala, resulted in HURP instability and complex disassembly. In addition, HURP-wild-type stable transfectants were capable of growing in low-serum environments whereas HURP-4P grew poorly under low-serum conditions and failed to proliferate. These studies together support the view that the ability to integrate evidence derived from microarray studies into biochemical analyses may ultimately augment our predictive power when analyzing the potential role of poorly characterized proteins. While this combined approach was simply an initial attempt to answer a range of complex biological questions, our findings do suggest that HURP is a potential oncogenic target of Aurora-A.
Figures
FIG. 1.
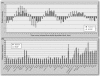
Similar expression patterns for _Aurora_-A and HURP from analysis of various data sets and regenerating mouse livers. The expression profiles for _Aurora_-A and HURP in a HeLa cell cycle panel (A) and in different cell lines and tissues (B) were downloaded from publicly accessible databases (two and one specific primers, respectively). The data were plotted according to the relative expression levels of the two genes. (C) Upregulation of _Aurora_-A expression parallels that of HURP in HCC. Q-RT-PCR was conducted with specific primers for either _Aurora_-A or HURP in tumor tissue or adjacent nontumorous analogs obtained from five HCC patients. The data were then normalized with the internal control, glyceraldehyde-3-phosphate dehydrogenase. TT, primary liver tumor tissue; NT, corresponding adjacent nontumorous liver tissue. (D) Expression of mouse _Aurora_-A and HURP during mouse liver regeneration was detected by Q-RT-PCR. β2-Microglobulin served as an internal control. Time indicates intervals after partial hepatectomy (h, hours; d, days). The left ordinate represents the _n_-fold change in mouse cyclin B2 mRNA. The right ordinate represents the _n_-fold change in mouse HURP and _Aurora_-A transcripts. The data were normalized with the expression value at time zero. The cell cycle stages, as determined by Q-RT-PCR of several markers (p21, PCNA, and cyclin B2) of each cell cycle stage, are shown at the bottom.
FIG. 1.
Similar expression patterns for _Aurora_-A and HURP from analysis of various data sets and regenerating mouse livers. The expression profiles for _Aurora_-A and HURP in a HeLa cell cycle panel (A) and in different cell lines and tissues (B) were downloaded from publicly accessible databases (two and one specific primers, respectively). The data were plotted according to the relative expression levels of the two genes. (C) Upregulation of _Aurora_-A expression parallels that of HURP in HCC. Q-RT-PCR was conducted with specific primers for either _Aurora_-A or HURP in tumor tissue or adjacent nontumorous analogs obtained from five HCC patients. The data were then normalized with the internal control, glyceraldehyde-3-phosphate dehydrogenase. TT, primary liver tumor tissue; NT, corresponding adjacent nontumorous liver tissue. (D) Expression of mouse _Aurora_-A and HURP during mouse liver regeneration was detected by Q-RT-PCR. β2-Microglobulin served as an internal control. Time indicates intervals after partial hepatectomy (h, hours; d, days). The left ordinate represents the _n_-fold change in mouse cyclin B2 mRNA. The right ordinate represents the _n_-fold change in mouse HURP and _Aurora_-A transcripts. The data were normalized with the expression value at time zero. The cell cycle stages, as determined by Q-RT-PCR of several markers (p21, PCNA, and cyclin B2) of each cell cycle stage, are shown at the bottom.
FIG. 2.
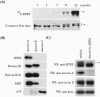
HURP is a potential substrate of Aurora-A. (A) Recombinant Aurora-A was incubated with recombinant His-tagged HURP in kinase reaction buffer in the presence of [γ-32P]ATP for different time intervals. The samples were separated by SDS-PAGE and transferred to a PVDF membrane, and this was followed by autoradiography (top) or membrane staining with Coomassie blue (bottom). An asterisk indicates multiple phosphorylation forms of HURP. (B) Recombinant Aurora-A, Aurora-B, and Aurora-C were incubated with recombinant His-tagged HURP in the presence of [γ-32P]ATP. Analysis of the autoradiography data demonstrates that Aurora-A efficiently phosphorylated the recombinant HURP; however, no such phosphorylation was observed when Aurora-B or Aurora-C was present. Histone H1, histone H2A, myelin basic protein (MBP), and p16 were also incubated with the Aurora kinases to serve as substrate controls and ensure that the input of Aurora-A kinase activity was similar to that of Aurora-B and that the Aurora-C used was active. (C) 293T cells were transfected with pSUPER vector (vehicle) or pSUPER-Aurora-A for 48 h and then synchronized in mitosis using nocodazole treatment for 16 h. Equal amounts of extracts were prepared and analyzed by Western blotting (WB) with anti-Aurora-A, anti-HURP, anti-cyclin B1, or anti-actin antibodies. Actin was used as a loading control. The similar cyclin B1 expression level indicates a comparable synchronization effect for pSUPER-Aurora-A transfectants during mitosis. The asterisk indicates the phosphorylation form of HURP.
FIG. 3.
Aurora-A regulates protein complex formation of HURP. 293T cells were transiently transfected with the pCMV2 vector (vehicle), FLAG-Aurora-A-WT, or FLAG-Aurora-A-KD. The cells were harvested and lysed. Equal amounts of lysates were subjected to protein liquid chromatography (FPLC). Fractions (tubes 32 to 56) were analyzed by SDS-PAGE, followed by Western blotting (WB) with anti-HURP antibody. Higher- and lower-molecular-weight fractions were also analyzed, and they contained essentially no HURP (data not shown). The lack of HURP signal in Aurora-A-KD-transfected cell lysates from tubes 32 to 44 was not due to differences in protein loading. The relative loading of each sample in the corresponding tubes was similar, as determined by visualization with Coomassie blue. The migration of the molecular size (MW) standards is shown at the top. Similar results were obtained for two independent transfections.
FIG.4.
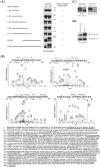
Aurora-A phosphorylates HURP at four residues in vitro. (A) HURP-WT and various deletion constructs were subjected to in vitro transcription and translation in the presence of [35S]methionine, followed by incubation with Aurora-A-WT or -KD, respectively. The phosphorylation status of HURPs was judged by mobility shift revealed by SDS-PAGE and autoradiography. (B) Recombinant HURPs were incubated with Aurora-A in kinase reaction buffer. After reaction at 30°C for 30 min, the samples were subjected to SDS-PAGE and stained with Coomassie blue. HURP bands were carefully sliced from the gel, and phosphorylation sites were determined by LC MS/MS analysis. Four residues were determined to be Aurora-A-dependent phosphorylation sites (the residues with asterisks above them in the upper four graphs) with 65% amino acid sequence coverage (the lower sequence; underlined amino acids represent those residues resolved by LC MS/MS). An asterisk indicates a phosphorylation site. (C) The four Aurora-A in vitro phosphorylation sites on HURP, which had been determined by LC MS/MS, were replaced with alanine (designated HURP-4P). 293T cells were transiently transfected with HA-HURP-WT or HA-HURP-4P. Twenty-four hours after transfection, the cells were lysed and immunoprecipitated (IP) with anti-HA antibody. Immunocomplexes were incubated with Aurora-A in the kinase reaction buffer in the presence of [γ-32P]ATP at 30°C for 30 min. Reaction mixtures were separated by SDS-PAGE and analyzed using Western blotting (WB) with anti-HA antibody (left) or autoradiography (right). The asterisk indicates the phosphorylation form of HURP. The HURP-4P mutant showed no [γ-32P]ATP incorporation. (D) 293T cells were transiently transfected with HA-HURP-WT or HA-HURP-4P. Twenty-four hours after transfection, the 293T cells were either left untreated (referred to as asynchronized) or synchronized in mitosis by nocodazole treatment for 16 h. Equal amounts of extracts were prepared and analyzed by Western blotting with anti-HA antibody. A mobility upshift band (indicated by the asterisk) was found for HA-HURP-WT, but not HA-HURP-4P, suggesting differences in protein phosphorylation. Similar results were obtained in two independent experiments.
FIG. 5.
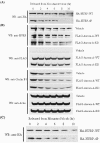
Aurora-A protects HURP from protein degradation. 293T cells were transiently transfected with HA-HURP-WT or HA-HURP-4P. Twenty-four hours after transfection, the 293T cells were synchronized in mitosis by nocodazole (A) or monastrol (C) treatment for 16 h. Cells were released into cell cycle progression by removing these mitosis-synchronizing agents and subsequently incubated with fresh medium containing cycloheximide, which blocks de novo protein synthesis. At the indicated time points, cells were harvested and analyzed by Western blotting (WB) with anti-HA antibody. (B) 293T cells were transiently transfected with the pCMV2 vector (vehicle), FLAG-Aurora-A-WT, or FLAG-Aurora-A-KD; synchronized in mitosis by nocodazole treatment; and released from nocodazole blockage as described for panel A. At the indicated time points, the cells were harvested and analyzed by Western blotting with antibodies against HURP, FLAG, cyclin B1, and actin. Cyclin B1 is well known to be degraded at the M/G1 transition, and the rapid decrease in cyclin B1 serves as a positive control showing that protein degradation under these conditions is working normally. Actin was used as a loading control. The results shown are representative of three independent experiments.
FIG. 6.
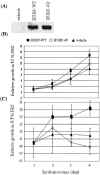
HURP-4P stable transfectant cannot proliferate in a low-serum environment. (A) 293T cell pooled stable clones expressing the vehicle, HA-HURP-WT, or HA-HURP-4P were established, and the protein expression levels of these clones were determined by Western blotting using anti-HA antibody. These stable transfectants (5 × 103) were seeded into 96-well plates with either 10% (B) or 0.5% (C) FBS for 1 to 4 days, followed by MTT assay (OD570) to quantify cell growth. Data were normalized against the OD570 value on day 1 for each pooled stable clone. The results are the averages of three independent assays.
Similar articles
- Identification of a novel cell cycle regulated gene, HURP, overexpressed in human hepatocellular carcinoma.
Tsou AP, Yang CW, Huang CY, Yu RC, Lee YC, Chang CW, Chen BR, Chung YF, Fann MJ, Chi CW, Chiu JH, Chou CK. Tsou AP, et al. Oncogene. 2003 Jan 16;22(2):298-307. doi: 10.1038/sj.onc.1206129. Oncogene. 2003. PMID: 12527899 - The involvement of nuclear factor-κappaB in the nuclear targeting and cyclin E1 upregulating activities of hepatoma upregulated protein.
Chen JM, Chiu SC, Wei TY, Lin SY, Chong CM, Wu CC, Huang JY, Yang ST, Ku CF, Hsia JY, Yu CT. Chen JM, et al. Cell Signal. 2015 Jan;27(1):26-36. doi: 10.1016/j.cellsig.2014.09.020. Epub 2014 Oct 5. Cell Signal. 2015. PMID: 25289861 - Aurora kinase inhibitors reveal mechanisms of HURP in nucleation of centrosomal and kinetochore microtubules.
Wu JM, Chen CT, Coumar MS, Lin WH, Chen ZJ, Hsu JT, Peng YH, Shiao HY, Lin WH, Chu CY, Wu JS, Lin CT, Chen CP, Hsueh CC, Chang KY, Kao LP, Huang CY, Chao YS, Wu SY, Hsieh HP, Chi YH. Wu JM, et al. Proc Natl Acad Sci U S A. 2013 May 7;110(19):E1779-87. doi: 10.1073/pnas.1220523110. Epub 2013 Apr 22. Proc Natl Acad Sci U S A. 2013. PMID: 23610398 Free PMC article. - Medical treatments: in association or alone, their role and their future perspectives: novel molecular-targeted therapy for hepatocellular carcinoma.
Tanaka S, Arii S. Tanaka S, et al. J Hepatobiliary Pancreat Sci. 2010 Jul;17(4):413-9. doi: 10.1007/s00534-009-0238-8. Epub 2009 Nov 26. J Hepatobiliary Pancreat Sci. 2010. PMID: 19941009 Review. - Aurora kinases, aneuploidy and cancer, a coincidence or a real link?
Giet R, Petretti C, Prigent C. Giet R, et al. Trends Cell Biol. 2005 May;15(5):241-50. doi: 10.1016/j.tcb.2005.03.004. Trends Cell Biol. 2005. PMID: 15866028 Review.
Cited by
- Knockdown of DLGAP5 suppresses cell proliferation, induces G2/M phase arrest and apoptosis in ovarian cancer.
Zhang H, Liu Y, Tang S, Qin X, Li L, Zhou J, Zhang J, Liu B. Zhang H, et al. Exp Ther Med. 2021 Nov;22(5):1245. doi: 10.3892/etm.2021.10680. Epub 2021 Sep 2. Exp Ther Med. 2021. PMID: 34539841 Free PMC article. - AURKA, DLGAP5, TPX2, KIF11 and CKAP5: Five specific mitosis-associated genes correlate with poor prognosis for non-small cell lung cancer patients.
Schneider MA, Christopoulos P, Muley T, Warth A, Klingmueller U, Thomas M, Herth FJ, Dienemann H, Mueller NS, Theis F, Meister M. Schneider MA, et al. Int J Oncol. 2017 Feb;50(2):365-372. doi: 10.3892/ijo.2017.3834. Epub 2017 Jan 2. Int J Oncol. 2017. PMID: 28101582 Free PMC article. - Inhibition of apoptosis by oncogenic hepatitis B virus X protein: Implications for the treatment of hepatocellular carcinoma.
Chao CC. Chao CC. World J Hepatol. 2016 Sep 8;8(25):1061-6. doi: 10.4254/wjh.v8.i25.1061. World J Hepatol. 2016. PMID: 27660672 Free PMC article. Review. - Aurora Kinase A expression predicts platinum-resistance and adverse outcome in high-grade serous ovarian carcinoma patients.
Mignogna C, Staropoli N, Botta C, De Marco C, Rizzuto A, Morelli M, Di Cello A, Franco R, Camastra C, Presta I, Malara N, Salvino A, Tassone P, Tagliaferri P, Barni T, Donato G, Di Vito A. Mignogna C, et al. J Ovarian Res. 2016 May 21;9(1):31. doi: 10.1186/s13048-016-0238-7. J Ovarian Res. 2016. PMID: 27209210 Free PMC article. - A quantitative atlas of mitotic phosphorylation.
Dephoure N, Zhou C, Villén J, Beausoleil SA, Bakalarski CE, Elledge SJ, Gygi SP. Dephoure N, et al. Proc Natl Acad Sci U S A. 2008 Aug 5;105(31):10762-7. doi: 10.1073/pnas.0805139105. Epub 2008 Jul 31. Proc Natl Acad Sci U S A. 2008. PMID: 18669648 Free PMC article.
References
- Alizadeh, A. A., M. B. Eisen, R. E. Davis, C. Ma, I. S. Lossos, A. Rosenwald, J. C. Boldrick, H. Sabet, T. Tran, X. Yu, J. I. Powell, L. Yang, G. E. Marti, T. Moore, J. J. Hudson, L. Lu, D. B. Lewis, R. Tibshirani, G. Sherlock, W. C. Chan, T. C. Greiner, D. D. Weisenburger, J. O. Armitage, R. Warnke, R. Levy, W. Wilson, M. R. Grever, J. C. Byrd, D. Botstein, P. O. Brown, and L. M. Staudt. 2000. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 403:503-511. - PubMed
- Anand, S., S. Penrhyn-Lowe, and A. R. Venkitaraman. 2003. AURORA-A amplification overrides the mitotic spindle assembly checkpoint, inducing resistance to Taxol. Cancer Cell 3:51-62. - PubMed
- Bar-Shira, A., J. H. Pinthus, U. Rozovsky, M. Goldstein, W. R. Sellers, Y. Yaron, Z. Eshhar, and A. Orr-Urtreger. 2002. Multiple genes in human 20q13 chromosomal region are involved in an advanced prostate cancer xenograft. Cancer Res. 62:6803-6807. - PubMed
- Bischoff, J. R., and G. D. Plowman. 1999. The Aurora/Ipl1p kinase family: regulators of chromosome segregation and cytokinesis. Trends Cell Biol. 9:454-459. - PubMed
- Bischoff, J. R., L. Anderson, Y. Zhu, K. Mossie, L. Ng, B. Souza, B. Schryver, P. Flanagan, F. Clairvoyant, C. Ginther, C. S. Chan, M. Novotny, D. J. Slamon, and G. D. Plowman. 1998. A homologue of Drosophila aurora kinase is oncogenic and amplified in human colorectal cancers. EMBO J. 17:3052-3065. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous