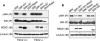Regulation of mTOR and cell growth in response to energy stress by REDD1 - PubMed (original) (raw)
Regulation of mTOR and cell growth in response to energy stress by REDD1
Avi Sofer et al. Mol Cell Biol. 2005 Jul.
Abstract
The tuberous sclerosis tumor suppressors TSC1 and TSC2 regulate the mTOR pathway to control translation and cell growth in response to nutrient and growth factor stimuli. We have recently identified the stress response REDD1 gene as a mediator of tuberous sclerosis complex (TSC)-dependent mTOR regulation by hypoxia. Here, we demonstrate that REDD1 inhibits mTOR function to control cell growth in response to energy stress. Endogenous REDD1 is induced following energy stress, and REDD1-/- cells are highly defective in dephosphorylation of the key mTOR substrates S6K and 4E-BP1 following either ATP depletion or direct activation of the AMP-activated protein kinase (AMPK). REDD1 likely acts on the TSC1/2 complex, as regulation of mTOR substrate phosphorylation by REDD1 requires TSC2 and is blocked by overexpression of the TSC1/2 downstream target Rheb but is not blocked by inhibition of AMPK. Tetracycline-inducible expression of REDD1 triggers rapid dephosphorylation of S6K and 4E-BP1 and significantly decreases cellular size. Conversely, inhibition of endogenous REDD1 by short interfering RNA increases cell size in a rapamycin-sensitive manner, and REDD1-/- cells are defective in cell growth regulation following ATP depletion. These results define REDD1 as a critical transducer of the cellular response to energy depletion through the TSC-mTOR pathway.
Figures
FIG. 1.
REDD1 is induced following energy stress and is required for mTOR substrate dephosphorylation. (A) Induction of the REDD1 message. Immortal MEFs of the indicated genotypes were washed, followed by addition of control medium with 10% fetal calf serum (C), the same medium/fetal calf serum with 2DG (25 mM), or glucose-free medium with 10% dialyzed fetal calf serum. Cells were harvested for Northern analysis at 4 h and probed with a REDD1 or REDD2 cDNA fragment. REDD2 expression is not detectable in MEFs, and no REDD1 expression is observed in _REDD1_−/− MEFs. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. (B) REDD1 wild-type and targeted genomic alleles. White boxes, REDD1 3′ UTR; black boxes, REDD1 coding region and 5′ UTR; grey box, β-galactosidase/neo fusion cDNA. The sizes of KpnI (K) fragments detected in panel C are shown. (C) Southern blot analysis of KpnI-digested MEF DNA of the indicated genotype, probed with a genomic fragment 3′ of the REDD1 targeting construct (not shown). (D) Absence of REDD1 protein in _REDD1_−/− MEFs. Extracts from cells treated with 2DG to induce REDD1 protein were analyzed by immunoprecipitation-Western analysis using affinity-purified polyclonal REDD1 antisera. The immunoglobulin heavy chain (HC) is shown as a control for immunoprecipitation. (E) Dephosphorylation of S6K (T389), S6 (S235/236), and 4EBP1 (T70) is defective in _REDD1_−/− MEFs following ATP depletion. Western blot analysis of lysates from primary MEFs treated with 2DG for 4 h, probed with phosphospecific antibodies. The same blots were stripped and reprobed to determine the respective total protein levels. (F) S6K dephosphorylation (T389) in response to glucose starvation requires REDD1. Glucose withdrawal in MEFs was performed as in panel A for the indicated times prior to harvest for Western blot analysis as above.
FIG. 2.
Signaling to mTOR substrates is dysregulated in _REDD1_−/− cells following AMPK, but not AKT, activation. (A) Downregulation of S6K (T389) and 4E-BP1 (T70) phosphorylation is defective in _REDD1_−/− cells following AMPK activation. Primary MEFs were serum starved (SS) for 24 h and then treated as indicated (S, serum) for 90 min prior to Western blot analysis. The phosphorylation forms of 4E-BP1 are indicated by convention as α, β, and γ (bottom panel) (19). Note in particular the absence of the α 4E-BP1 form in _REDD1_−/− cells (lane 5 versus 10). (B) Reconstitution of REDD1 expression restores S6K (T389) and 4E-BP1 (T70) dephosphorylation following AMPK activation (lanes 4 and 8). Primary _REDD1_−/− MEFs were infected with control or REDD1-expressing retrovirus prior to treatment as in panel A for 90 min. (C) REDD1 reconstitution restores 4E-BP1 (T70) dephosphorylation following ATP depletion. Primary _REDD1_−/− MEFs were retrovirally infected as in panel B and then treated with 2DG as indicated for 4 h.
FIG. 3.
REDD1 is not required for AKT or AMPK activation. (A) AMPK (T172) is phosphorylated following AICAR treatment in both wild-type and _REDD1_−/− MEFs (last two lanes). Primary MEFs of the indicated genotype were serum starved for 24 h (SS) and then treated as indicated (S, serum) for 90 min prior to Western blot analysis. (B) AMPK is functionally activated by ATP depletion in both wild-type and _REDD1_−/− MEFs. Primary MEFs were treated with 2DG (50 mM) for the indicated times, prior to Western blot analysis for phospho-AMPK (T172) or its substrate phospho-ACC (S79). (C) AMPK-dependent phosphorylation of TSC2 does not require REDD1. MEFs were serum starved as in panel A, pretreated with the AMPK inhibitor (AMPKI) compound C (10 μM) for 30 min where indicated, and then treated with AICAR (2.0 mM) for 30 min prior to Western blotting for endogenous TSC2. The phosphorylation-induced shift of TSC2 (upper versus lower arrowhead) is evident compared to the uniform migration of the nonspecific band (Non-Spec.). TSC2 phosphorylation correlates with AMPK activation, as evidenced by ACC (S79) phosphorylation.
FIG. 4.
REDD1 function requires TSC2 and is suppressed by Rheb expression. (A) REDD1 regulation of S6K (T389) phosphorylation requires TSC2. Fibroblasts of the indicated genotype were transfected with HA-S6K, with or without REDD1 (R1) or REDD1-dC (dC) (1:1 molar ratio), treated as indicated with rapamycin (Rapa, 2 nM, 90 min), and then immunoprecipitated with anti-HA, followed by Western blot analysis (IP). Following phospho-S6K analysis, the same membrane was stripped and reprobed for total S6K. Four percent of the lysate was probed for REDD1 or β-tubulin as a loading control (IB). (B) Rheb suppresses regulation of S6K (T389) phosphorylation by REDD1. Fibroblasts were transfected with S6K; S6K plus REDD1, REDD1-dC, or Rheb (2:1 molar ratio); or S6K plus REDD1 and Rheb (2:1:1 ratio) and then treated with rapamycin and immunoprecipitated as in panel A. Four percent of the lysate was probed for REDD1 or loading control as in panel A.
FIG. 5.
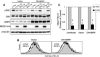
REDD1 regulation of mTOR substrate phosphorylation decreases cell size. (A) REDD1, but not REDD1-dC, downregulates phosphorylation of S6K (T389), S6 (S235/236), and 4E-BP1 (T70). Expression of REDD1-dC or REDD1 was induced by addition of tetracycline (1 μg/ml) for the indicated time, and lysates were analyzed by Western blot analysis. The α, β, and γ phosphorylation forms of 4E-BP1 are indicated. (B) Cell cycle profiles of U2-REDD1 cells treated with tetracycline as in panel A or with rapamycin (Rapa, 20 nM) for 48 h and then fixed and stained with PI and analyzed by flow cytometry. (C) REDD1 induction decreases cell size. Representative histogram showing cell size distribution (FSC-H) of G1-gated U2-REDD1 cells treated as in panel B for 48 h (black line) or untreated (grey shading) and then stained with PI and analyzed by flow cytometry. (D) Quantitation of cell size decrease following REDD1 induction. The average of mean FSC-H values from five independent experiments is shown for U2-REDD1 or U2-REDD1-dC cells treated and analyzed as in panel C. *, P = 0.001 versus (−) Tet. Error bars show 1 standard deviation.
FIG. 6.
AMPK activation is not required for REDD1 function. (A) DN-AMPK blocks phospho-S6K (T389) regulation by 2DG but not by REDD1. U2-REDD1 cells were infected with DN-AMPK or vector control adenovirus, and 24 h later cells were treated with tetracycline (Tet) to induce REDD1 or with 2DG (25 mM) for the indicated times (T4 and T8, 4 and 8 h of treatment, respectively). Note that DN-AMPK comigrates with endogenous AMPK. (B) DN-AMPK does not inhibit cell size regulation by REDD1. Histogram showing cell size (FSC-H) distribution of G1-gated U2-REDD1 cells infected as in panel A and then induced to express REDD1 (black line) or untreated (grey shading) for 48 h. (C) Quantitation of cell size decrease following induction of REDD1, which is unchanged in the presence or absence of DN-AMPK. The average of mean FSC-H values is shown for three independent experiments performed as in panel B. *, P < 0.005 versus (−)Tet for all comparisons. Error bars show 1 standard deviation.
FIG. 7.
Endogenous REDD1 regulates cell size in response to energy stress. (A) Efficient inhibition of REDD1 by RNAi. Western blot showing REDD1-HA expression in U2OS cells transfected with REDD1-HA along with the indicated siRNA-expressing plasmid, the backbone vector (V), or a nonspecific siRNA (siScr). (B) REDD1 RNAi increases cell size. Histogram showing cell size distribution (FSC-H) of G1-gated U2OS cells expressing the indicated siRNA (black lines) or control vector plasmid (grey shading), 48 h following transfection (see Materials and Methods). (C) Increased cell size following REDD1 RNAi is reversed following rapamycin treatment. Quantitation of cell size (average of mean FSC-H values) for U2OS cells 48 h following transfection with the indicated siRNA construct, analyzed as in panel B. Where indicated, rapamycin (20 nM) was added 12 h following transfection. Mean values for four independent experiments are shown. *, P < 0.002 versus vector; **, P = NS versus vector. (D) REDD1 is required for cell size regulation following ATP depletion. Histogram showing cell size distribution (FSC-H) of G1-gated MEFs, treated with 2DG (2 mM) for 48 h. (E) Quantitation of altered cell size regulation in _REDD1_−/− MEFs following ATP depletion. The average of mean FSC-H values is shown for four independent experiments. *, P = 0.02 for wild-type versus _REDD1_−/− cells. Error bars show 1 standard deviation.
FIG. 8.
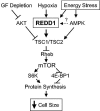
Proposed model of REDD1 function in response to energy stress. Energy stress induces expression of REDD1, which functions in a TSC-dependent manner upstream of Rheb to inhibit mTOR activity. REDD1 induction therefore promotes dephosphorylation of S6K and 4E-BP1, which inhibits protein synthesis and decreases cellular growth/cell size. GF, growth factor.
Similar articles
- Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex.
Brugarolas J, Lei K, Hurley RL, Manning BD, Reiling JH, Hafen E, Witters LA, Ellisen LW, Kaelin WG Jr. Brugarolas J, et al. Genes Dev. 2004 Dec 1;18(23):2893-904. doi: 10.1101/gad.1256804. Epub 2004 Nov 15. Genes Dev. 2004. PMID: 15545625 Free PMC article. - Loss of tuberous sclerosis complex 1 (Tsc1) expression results in increased Rheb/S6K pathway signaling important for astrocyte cell size regulation.
Uhlmann EJ, Li W, Scheidenhelm DK, Gau CL, Tamanoi F, Gutmann DH. Uhlmann EJ, et al. Glia. 2004 Aug 1;47(2):180-8. doi: 10.1002/glia.20036. Glia. 2004. PMID: 15185396 - Growth control under stress: mTOR regulation through the REDD1-TSC pathway.
Ellisen LW. Ellisen LW. Cell Cycle. 2005 Nov;4(11):1500-02. doi: 10.4161/cc.4.11.2139. Epub 2005 Nov 1. Cell Cycle. 2005. PMID: 16258273 Review. - Dexamethasone represses signaling through the mammalian target of rapamycin in muscle cells by enhancing expression of REDD1.
Wang H, Kubica N, Ellisen LW, Jefferson LS, Kimball SR. Wang H, et al. J Biol Chem. 2006 Dec 22;281(51):39128-34. doi: 10.1074/jbc.M610023200. Epub 2006 Oct 30. J Biol Chem. 2006. PMID: 17074751 - The mTOR/S6K signalling pathway: the role of the TSC1/2 tumour suppressor complex and the proto-oncogene Rheb.
Nobukini T, Thomas G. Nobukini T, et al. Novartis Found Symp. 2004;262:148-54; discussion 154-9, 265-8. Novartis Found Symp. 2004. PMID: 15562827 Review.
Cited by
- Targeting the mTOR-DEPTOR pathway by CRL E3 ubiquitin ligases: therapeutic application.
Zhao Y, Sun Y. Zhao Y, et al. Neoplasia. 2012 May;14(5):360-7. doi: 10.1593/neo.12532. Neoplasia. 2012. PMID: 22745582 Free PMC article. Review. - Autophagy, a process within reperfusion injury: an update.
Thapalia BA, Zhou Z, Lin X. Thapalia BA, et al. Int J Clin Exp Pathol. 2014 Dec 1;7(12):8322-41. eCollection 2014. Int J Clin Exp Pathol. 2014. PMID: 25674198 Free PMC article. Review. - Silymarin Suppresses Cellular Inflammation By Inducing Reparative Stress Signaling.
Lovelace ES, Wagoner J, MacDonald J, Bammler T, Bruckner J, Brownell J, Beyer RP, Zink EM, Kim YM, Kyle JE, Webb-Robertson BJ, Waters KM, Metz TO, Farin F, Oberlies NH, Polyak SJ. Lovelace ES, et al. J Nat Prod. 2015 Aug 28;78(8):1990-2000. doi: 10.1021/acs.jnatprod.5b00288. Epub 2015 Jul 17. J Nat Prod. 2015. PMID: 26186142 Free PMC article. - RTP801 is a novel retinoic acid-responsive gene associated with myeloid differentiation.
Gery S, Park DJ, Vuong PT, Virk RK, Muller CI, Hofmann WK, Koeffler HP. Gery S, et al. Exp Hematol. 2007 Apr;35(4):572-8. doi: 10.1016/j.exphem.2007.01.049. Exp Hematol. 2007. PMID: 17379067 Free PMC article. - The hypoxia-inducible factor HIF-1 functions as both a positive and negative modulator of aging.
Leiser SF, Kaeberlein M. Leiser SF, et al. Biol Chem. 2010 Oct;391(10):1131-7. doi: 10.1515/BC.2010.123. Biol Chem. 2010. PMID: 20707608 Free PMC article. Review.
References
- Arsham, A. M., J. J. Howell, and M. C. Simon. 2003. A novel hypoxia-inducible factor-independent hypoxic response regulating mammalian target of rapamycin and its targets. J. Biol. Chem. 278:29655-29660. - PubMed
- Brafman, A., I. Mett, M. Shafir, H. Gottlieb, G. Damari, S. Gozlan-Kelner, V. Vishnevskia-Dai, R. Skaliter, P. Einat, A. Faerman, E. Feinstein, and T. Shoshani. 2004. Inhibition of oxygen-induced retinopathy in RTP801-deficient mice. Investig. Ophthalmol. Vis. Sci. 45:3796-3805. - PubMed
- Cantley, L. C. 2002. The phosphoinositide 3-kinase pathway. Science 296:1655-1657. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous