Nuclear PTEN-mediated growth suppression is independent of Akt down-regulation - PubMed (original) (raw)
Nuclear PTEN-mediated growth suppression is independent of Akt down-regulation
Juinn-Lin Liu et al. Mol Cell Biol. 2005 Jul.
Abstract
The tumor suppressor gene PTEN is a phosphoinositide phosphatase that is inactivated by deletion and/or mutation in diverse human tumors. Wild-type PTEN is expressed both in the cytoplasm and nucleus in normal cells, with a preferential nuclear localization in differentiated or resting cells. To elucidate the relationship between PTEN's subcellular localization and its biologic activities, we constructed different PTEN mutants that targeted PTEN protein into different subcellular compartments. Our data show that the subcellular localization patterns of a PTEN (deltaPDZB) mutant versus a G129R phosphatase mutant were indistinguishable from those of wild-type PTEN. In contrast, the Myr-PTEN mutant demonstrated an enhanced association with the cell membrane. We found that nuclear PTEN alone is capable of suppressing anchorage-independent growth and facilitating G1 arrest in U251MG cells without inhibiting Akt activity. Nuclear compartment-specific PTEN-induced growth suppression is dependent on possessing a functional lipid phosphatase domain. In addition, the down-regulation of p70S6K could be mediated, at least in part, through activation of AMP-activated protein kinase in an Akt-independent fashion. Introduction of a constitutively active mutant of Akt, Akt-DD, only partially rescues nuclear PTEN-mediated growth suppression. Our collective results provide the first direct evidence that PTEN can contribute to G1 growth arrest through an Akt-independent signaling pathway.
Figures
FIG. 1.
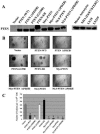
Schematic diagram of PTEN localization mutants. To target PTEN into different subcellular compartments, specific localization signals were inserted into PTEN′s sequence via PCR or molecular cloning as described in Materials and Methods. These were: N-terminal Myr (8-amino-acid [aa] myristoylation motif, MKGSLTTH) targeting the plasma membrane, N-terminal NLS (7 aa, M+RRKKRK) targeting the nucleus, and ER retention motifs (21 N-terminal aa containing an ER signal peptide and 28 C-terminal aa containing an ER retention signal) targeting the ER. In addition, TKV/TVD mutations were introduced to abolish PTEN′s PDZ binding ability.
FIG. 2.
Subcellular localization of PTEN mutants. To examine subcellular localization of PTEN mutants, U251MG cells were transfected with expression vectors for the mutants shown in Fig. 1 and clonal populations were obtained as described in Materials and Methods. Immunostaining was performed with a MAb to PTEN, followed by confocal microscopy, as described. Differential interference contrast was used to depict cellular contrast; in the case of PTEN-ER, localization in the ER was verified by successful transfection of the pCMV-GFP-ER construct.
FIG. 3.
Substantiation of PTEN′s localization with subcellular fractionation. Immunoblotting with a MAb to PTEN was performed on cellular fractions extracted from cell lines expressing WT PTEN and U251MG PTEN mutant clones, as indicated (C, cytoplasm; N, nucleus), to corroborate the immunostaining results. Immunoblotting with MAb against poly(ADP-ribose) polymerase 1 (PARP-1), a nucleus-specific protein, showed minimum cross-contamination between the cytoplasmic and nuclear fractions.
FIG. 4.
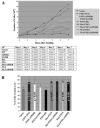
Nuclear PTEN is capable of suppressing anchorage-independent growth. (A) PTEN localization mutants expressing comparable protein levels as determined by immunoblotting were selected for functional analysis, as described in Materials and Methods. The migration of the PTEN-ER protein was slower than the other PTEN mutants due to the larger size of ER retention motifs, as illustrated in Fig. 1. (B) The effect of PTEN and its mutants on anchorage-independent growth was assessed using a soft agar colony assay. Selected stable U251MG transfectants were seeded into six-well plates in duplicate at a concentration of 5 × 104/well in the presence of 1% serum. (C) The number of colonies was scored under a microscope after 2 weeks. The results shown are the averages of the results from three experiments (means ± SD). An unpaired (equal variance) t test was performed on all PTEN and PTEN mutant clones compared to the vector control. The P values of PTEN (WT), PTEN (ΔPDZB), Myr, Myr (ΔPDZB), NLS, and NLS (ΔPDZB) clones are statistically significant (*, P < 10−5). In addition, the size of their soft agar colonies appeared significantly smaller than those of vector colonies.
FIG. 5.
Nuclear PTEN-induced growth suppression is mediated through facilitating G1 accumulation. (A) Proliferation rates of cells expressing PTEN mutants. Equal numbers of cells (1 × 105) were seeded in 60-mm dishes in the presence of 1% serum, and cells were subsequently counted at days 2 through 7 after seeding. The results shown are the averages of the results from four separate experiments (means ± SD). (B) G1 arrest induced by PTEN mutants. Stable U251MG transfectants were maintained in medium containing 1% serum for 3 days and collected for analysis of the cell cycle profile using flow cytometry. The results shown are the averages of the results from five separate experiments (means ± SD).
FIG. 6.
Nuclear PTEN does not inhibit cell invasion. (A) A Matrigel invasion assay was performed using a transwell membrane coated with 0.7 mg/ml of Matrigel matrix. Cells were seeded at a concentration of 2 × 105 cells/well overnight. Cells that invaded the bottom side of the membrane were fixed and stained using a hema-3 kit. (B) The number of invaded cells in vector clones was set at 100%. The results shown are the averages of the results from five individual experiments (means ± SD). Only PTEN (WT), ΔPDZB, Myr, and Myr (ΔPDZB) clones showed statistically significant inhibition of cell invasion compared to the vector control (*, P < 10−6).
FIG. 7.
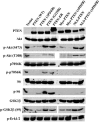
Nuclear PTEN inhibits p70S6K activation without down-regulating Akt. Cell lysates equivalent to 5 × 105 cells from stable clones grown in the presence of 1% serum for 3 days were loaded into each well on an sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel and transferred onto PVDF membranes. The filters were blotted with antibodies against PTEN, Akt, phospho-Akt (S473), phospho-Akt (T308), p70S6K, phospho-p70S6K (T389), S6, phospho-S6 (S235/236), GSK3β, phospho-GSK3β (S9), and phospho-Erk1/2, respectively, followed by horseradish peroxidase-conjugated secondary antibodies and visualized by the ECL reaction.
FIG. 8.
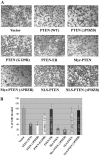
Assessment of in vivo lipid phosphatase activity of PTEN mutants. Stable transfectants were first immunostained with MAb against PTEN and then with FITC-conjugated goat anti-mouse IgG, followed by mouse IgM anti-PIP2 and then Texas red-conjugated goat anti-mouse IgM (μ chain) to avoid cross-reactivities of secondary antibodies. The fluorescence signals were analyzed by an Olympus FluoView LSM confocal microscope.
FIG. 9.
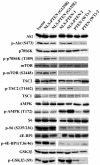
AMPK is activated by nuclear PTEN. Western blotting was performed on lysates of cells expressing the indicated mutants as described in Materials and Methods using antibodies against Akt, phospho-Akt (S473), p70S6K, phospho-p70S6K (T389), mTOR, phospho-mTOR (S2448), TSC2, phospho-TSC2 (T1462), TSC1, AMPK, phospho-AMPK (T172), S6, phospho-S6 (S235/236), 4E-BP1, phospho-4E-BP1 (T36/46), GSK3β, and phospho-GSK3β (S9), followed by horseradish peroxidase-conjugated secondary antibodies, and visualized by the ECL reaction.
FIG. 10.
Regulation of AMPK phosphorylation in U251MG cells. U251MG cells were either serum starved for 3 days and released in 10% serum or treated with 10 μM PD98059, 20 μM LY294002, 10 μM carbonyl cyanide _m_-chlorophenylhydrazone (CCCP), and 2 mM 5-aminoimidazole-4-carbozamide-1-β-4 ribofuranoside (AICAR) in the presence of 10% serum overnight. Cell lysates were subsequently collected to perform immunoblotting using antibodies against AMPK and phospho-AMPK (T172), followed by horseradish peroxidase-conjugated secondary antibodies, and visualized by the ECL reaction.
FIG. 11.
Phosphorylation of S6 remains suppressed by nuclear PTEN in the presence of Akt-DD. Cell lysates were collected from U251MG clones expressing the pLNCX vector, NLS-PTEN, Akt-DD, or NLS-PTEN plus Akt-DD grown in the presence of 1% serum. Western blotting was performed using antibodies against PTEN, HA tag, S6, and phospho-S6, followed by horseradish peroxidase-conjugated secondary antibodies, and visualized by the ECL reaction.
FIG. 12.
Nuclear PTEN down-regulates mTOR/p70S6K, bypassing PI3K/Akt signaling pathways. Despite the fact that mTOR-mediated p70S6K phosphorylation at the T389 residue is regulated primarily by Akt through TSC2 phosphorylation, other pathways have also been shown to regulate mTOR and/or p70S6K activities. Accordingly, nuclear PTEN can down-regulate mTOR/p70S6K through alternative mechanisms without interfering with Akt activation.
Similar articles
- PTEN induces G(1) cell cycle arrest and decreases cyclin D3 levels in endometrial carcinoma cells.
Zhu X, Kwon CH, Schlosshauer PW, Ellenson LH, Baker SJ. Zhu X, et al. Cancer Res. 2001 Jun 1;61(11):4569-75. Cancer Res. 2001. PMID: 11389092 - Loss of tumor suppressor p53 decreases PTEN expression and enhances signaling pathways leading to activation of activator protein 1 and nuclear factor kappaB induced by UV radiation.
Wang J, Ouyang W, Li J, Wei L, Ma Q, Zhang Z, Tong Q, He J, Huang C. Wang J, et al. Cancer Res. 2005 Aug 1;65(15):6601-11. doi: 10.1158/0008-5472.CAN-04-4184. Cancer Res. 2005. PMID: 16061640 - PTEN suppresses breast cancer cell growth by phosphatase activity-dependent G1 arrest followed by cell death.
Weng LP, Smith WM, Dahia PL, Ziebold U, Gil E, Lees JA, Eng C. Weng LP, et al. Cancer Res. 1999 Nov 15;59(22):5808-14. Cancer Res. 1999. PMID: 10582703 - Pten signaling in gliomas.
Knobbe CB, Merlo A, Reifenberger G. Knobbe CB, et al. Neuro Oncol. 2002 Jul;4(3):196-211. Neuro Oncol. 2002. PMID: 12084351 Free PMC article. Review. - Biological role of phosphatase PTEN in cancer and tissue injury healing.
Tsugawa K, Jones MK, Sugimachi K, Sarfeh IJ, Tarnawski AS. Tsugawa K, et al. Front Biosci. 2002 May 1;7:e245-51. doi: 10.2741/tsugawa. Front Biosci. 2002. PMID: 11991859 Review.
Cited by
- SUMO1 modification of PTEN regulates tumorigenesis by controlling its association with the plasma membrane.
Huang J, Yan J, Zhang J, Zhu S, Wang Y, Shi T, Zhu C, Chen C, Liu X, Cheng J, Mustelin T, Feng GS, Chen G, Yu J. Huang J, et al. Nat Commun. 2012 Jun 19;3:911. doi: 10.1038/ncomms1919. Nat Commun. 2012. PMID: 22713753 - Decrease in PTEN and increase in Akt expression and neuron size in aged rat spinal cord.
Rodrigues de Amorim MA, Garcia-Segura LM, Goya RG, Portiansky EL. Rodrigues de Amorim MA, et al. Exp Gerontol. 2010 Jun;45(6):457-63. doi: 10.1016/j.exger.2010.03.015. Epub 2010 Mar 27. Exp Gerontol. 2010. PMID: 20347952 Free PMC article. - PTEN in the maintenance of genome integrity: From DNA replication to chromosome segregation.
Hou SQ, Ouyang M, Brandmaier A, Hao H, Shen WH. Hou SQ, et al. Bioessays. 2017 Oct;39(10):10.1002/bies.201700082. doi: 10.1002/bies.201700082. Epub 2017 Sep 11. Bioessays. 2017. PMID: 28891157 Free PMC article. Review. - Germline and somatic cancer-associated mutations in the ATP-binding motifs of PTEN influence its subcellular localization and tumor suppressive function.
Lobo GP, Waite KA, Planchon SM, Romigh T, Nassif NT, Eng C. Lobo GP, et al. Hum Mol Genet. 2009 Aug 1;18(15):2851-62. doi: 10.1093/hmg/ddp220. Epub 2009 May 20. Hum Mol Genet. 2009. PMID: 19457929 Free PMC article. - Pten regulates development and lactation in the mammary glands of dairy cows.
Wang Z, Hou X, Qu B, Wang J, Gao X, Li Q. Wang Z, et al. PLoS One. 2014 Jul 10;9(7):e102118. doi: 10.1371/journal.pone.0102118. eCollection 2014. PLoS One. 2014. PMID: 25009983 Free PMC article.
References
- Adachi, J., K. Ohbayashi, T. Suzuki, and T. Sasaki. 1999. Cell cycle arrest and astrocytic differentiation resulting from PTEN expression in glioma cells. J. Neurosurg. 9:822-830. - PubMed
- Adey, N. B., L. Huang, P. A. Ormonde, M. L. Baumgard, R. Pero, D. V. Byreddy, S. V. Tavtigian, and P. L. Bartel. 2000. Threonine phosphorylation of the MMAC1/PTEN PDZ binding domain both inhibits and stimulates PDZ binding. Cancer Res. 60:35-37. - PubMed
- Belham, C., M. J. Comb, and J. Avruch. 2001. Identification of the NIMA family kinases NEK6/7 as regulators of the p70 ribosomal S6 kinase. Curr. Biol. 11:1155-1167. - PubMed
- Berven, L. A., and M. F. Crouch. 2000. Cellular function of p70S6K: a role in regulating cell motility. Immunol. Cell Biol. 78:447-451. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous