Arachidonate-regulated Ca2+-selective (ARC) channel activity is modulated by phosphorylation and involves an A-kinase anchoring protein - PubMed (original) (raw)
Arachidonate-regulated Ca2+-selective (ARC) channel activity is modulated by phosphorylation and involves an A-kinase anchoring protein
Olivier Mignen et al. J Physiol. 2005.
Abstract
In many non-excitable cells, the predominant mode of agonist-activated Ca(2+) entry switches from the arachidonic acid-regulated Ca(2+) (ARC) channels at low agonist concentrations, to store-operated channels at high concentrations. Underlying this process is the inhibition of the ARC channels by a calcineurin-mediated dephosphorylation, which inhibits the ability of arachidonic acid to activate the channels. Following such a dephosphorylation, we found that restoration of the sensitivity of the ARC channels to arachidonic acid, as well as to low concentrations of carbachol, was specifically dependent on protein kinase A (PKA) activity. Inhibition of protein kinase C, protein kinase G or calmodulin-activated kinase had no effect. This action of PKA was unaffected by prolonged intracellular dialysis, whilst disruption of the binding of PKA to A-kinase anchoring proteins (AKAPs) inhibited currents through ARC channels, and blocked the PKA-dependent effects. AKAP79, a protein which scaffolds both PKA and calcineurin, was shown to be present in the cells. These data illustrate the significance of PKA-dependent phosphorylation and calcineurin-dependent dephosphorylation in the overall regulation of ARC channel activity, and indicate the key role of an AKAP, possibly AKAP79, in the spatial organization these processes.
Figures
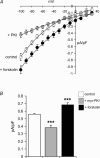
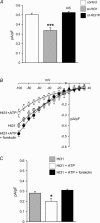
Figure 1. Inhibition of PKA-dependent phosphorylation reduces ARC channel activity
The effect of forskolin (10 μ
m
) and incubation in the presence of the PKA inhibitor myr-PKI (20 μ
m
for 10–20 min) on the I–V relationship (A) and the mean inward currents measured at −80 mV (B) from cells activated by addition of 8 μ
m
arachidonic acid. For the I–V curves: control cells, n = 5; myr-PKI-treated cells, n = 4 forskolin-treated cells, n = 6; for mean inward currents: control cells, n = 20; myr-PKI-treated cells, n = 12; forskolin-treated cells, n = 10. ***P < 0.001.
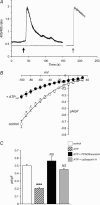
Figure 2. Activation of endogenous P2Y receptors with external ATP inhibits the currents through ARC channels
A, representative trace showing that addition of 100 μ
m
ATP (added at the black arrow) induces an increase in [Ca2+]i as measured by intracellularly loaded indo-1. This increase is blocked by the P2Y receptor antagonists PPADS (100 μ
m
) and suramin (50 μ
m
) (grey trace). However, addition of these antagonists does not affect the [Ca2+]i signal induced by addition of the muscarinic agonist carbachol (10 μ
m
) (added at grey arrow). B, the arachidonic acid-activated (8 μ
m
) I–V curves from control cells, and from cells exposed to 100 μ
m
ATP for 5 min (n = 5 in both cases). C, the effects of exposure to ATP (100 μ
m
) on the mean inward arachidonic acid-activated currents measured at −80 mV in control cells, and in cells pretreated with PPADS plus suramin (100 μ
m
and 50 μ
m
, respectively) or cyclosporin A (1 μ
m
for 15–45 min). control cells, n = 7; cells exposed to ATP, n = 13; cells treated with PPADS and suramin, n = 5; cyclosporin-treated cells, n = 12. ***P < 0.001 versus control; NS, not significantly different versus control.
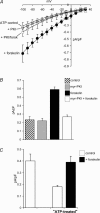
Figure 3. PKA-dependent phosphorylation modulates ARC channel activity following ATP-pretreatment
The effect of forskolin (10 μ
m
) and incubation in the presence of the PKA inhibitor myr-PKI (20 μ
m
for 10–20 min) on the I–V curves (A) and the mean inward currents measured at −80 mV (B) from ATP-pretreated cells activated by addition of 8 μ
m
arachidonic acid. For the I–V curves: ATP-treated control cells, n = 7; myr-PKI-treated cells, n = 5; forskolin-treated cells, n = 5; myr-PKI cell treated with forskolin, n = 4. For the mean inward currents: control cells, n = 6; myr-PKI-treated cells both alone and with forskolin, n = 4; forskolin-treated cells, n = 8. C, the effect of ATP-pretreatment (100 μ
m
for 5 min) and the subsequent addition of forskolin (10 μ
m
) on the mean inward currents measured at −80 mV in cells activated by addition of a low concentration (0.5 μ
m
) of carbachol. Control, n = 3; ATP-pretreated cells, n = 7; forskolin-treated cells, n = 5.
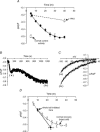
Figure 4. Following dephosphorylation, a spontaneous PKA-dependent rephosphorylation reactivates ARC channels
A, cells were exposed to ATP (100 μ
m
) and then subsequently whole-cell patch clamped at the times indicated and the arachidonic acid-activated currents at −80 mV determined (•). The normal mean arachidonic acid-activated currents measured in control cells (□, n = 7) and after 5 min of ATP exposure (100 μ
m
; ○, n = 13) are shown. The mean arachidonic acid-activated current in ATP-treated cells that were continuously incubated in the presence of myr-PKI (20 μ
m
) for the time indicated before whole-cell patch clamping is shown (▵, n = 9). B, representative trace of an experiment showing the progressive development of the inward arachidonic acid-activated current in a cell maintained under whole-cell patch-clamp conditions. The cell was treated with 100 μ
m
ATP and 6 min later whole-cell patch clamp was initiated. Addition of arachidonic acid (8 μ
m
) at time zero induced a rapid development of the inward current measured at −80 mV (a). This current then gradually increases to reach a stable value after an additional approximately 18 min (b). C, individual I–V curves for the cell shown in (B) taken at points a and b. The data are plotted as a five-point moving average for clarity. D, mean data from experiments similar to that shown in B. Cells (n = 4) were exposed to ATP (100 μ
m
) at time zero. Ten min later whole-cell patch-clamp conditions were initiated, and the arachidonic acid-activated inward currents determined (first filled circle). Whole-cell patch-clamp conditions were maintained until the arachidonic acid-activated currents stabilized, and the mean value of that current and the time at which it was achieved are indicated (second filled circle). The data for intact cells (see A above) are included for comparison.
Figure 5. The effects of phosphorylation on ARC channel activity involve an AKAP
A, mean inward arachidonic acid-activated currents measured at −80 mV in control cells (n = 7), in cells treated with st-Ht31 (n = 8) and in cells treated with the control peptide st-Ht31P (n = 6). ***P < 0.001 versus control. Mean I–V curves (B) and mean inward currents measured at −80 mV (C) for the arachidonic acid-activated current in cells patch clamped with a pipette solution containing Ht31 (30 μ
m
), in such cells subsequently exposed to ATP (100 μ
m
for 5 min) and following addition of forskolin (10 μ
m
). For the I–V curves: Ht31 cells, n = 4; ATP-treated cells, n = 6; addition of forskolin, n = 3. For the mean inward currents: Ht31 cells, n = 5; ATP-treated cells, n = 10; addition of forskolin, n = 6. *P < 0.05 versus Ht31 control; NS, not significant versus Ht31 control.
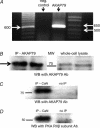
Figure 6. AKAP79 is present in the m3-HEK cells
A, results from a one-step RT-PCR reaction performed on total RNA from cells using primers specific for AKAP79. The predicted RT-PCR product was 635 base pairs. The negative control was the same reaction carried out in the absence of reverse transcriptase. B, representative Western blot showing the immunoreaction of a crude cell membrane preparation immunoprecipitated with an antibody to AKAP79 and blotted with the same antibody. For comparison, a whole-cell lysate blotted with the AKAP79 antibody is shown. The immunoreaction of a crude cell membrane preparation immunoprecipitated with an antibody to calcineurin and then blotted with an AKAP79 antibody (C) or with an antibody to the RIIβ subunit of PKA (D).
Similar articles
- Agonist activation of arachidonate-regulated Ca2+-selective (ARC) channels in murine parotid and pancreatic acinar cells.
Mignen O, Thompson JL, Yule DI, Shuttleworth TJ. Mignen O, et al. J Physiol. 2005 May 1;564(Pt 3):791-801. doi: 10.1113/jphysiol.2005.085704. Epub 2005 Mar 10. J Physiol. 2005. PMID: 15760932 Free PMC article. - AKAP79/150 anchoring of calcineurin controls neuronal L-type Ca2+ channel activity and nuclear signaling.
Oliveria SF, Dell'Acqua ML, Sather WA. Oliveria SF, et al. Neuron. 2007 Jul 19;55(2):261-75. doi: 10.1016/j.neuron.2007.06.032. Neuron. 2007. PMID: 17640527 Free PMC article. - Regulation of Ca2+-dependent desensitization in the vanilloid receptor TRPV1 by calcineurin and cAMP-dependent protein kinase.
Mohapatra DP, Nau C. Mohapatra DP, et al. J Biol Chem. 2005 Apr 8;280(14):13424-32. doi: 10.1074/jbc.M410917200. Epub 2005 Feb 3. J Biol Chem. 2005. PMID: 15691846 - ARC channels: a novel pathway for receptor-activated calcium entry.
Shuttleworth TJ, Thompson JL, Mignen O. Shuttleworth TJ, et al. Physiology (Bethesda). 2004 Dec;19:355-61. doi: 10.1152/physiol.00018.2004. Physiology (Bethesda). 2004. PMID: 15546853 Review. - The role of cyclic AMP-dependent phosphorylation in the maintenance and modulation of voltage-activated calcium channels.
Chad J, Kalman D, Armstrong D. Chad J, et al. Soc Gen Physiol Ser. 1987;42:167-86. Soc Gen Physiol Ser. 1987. PMID: 2850609 Review.
Cited by
- Acid-sensing ion channels in pathological conditions.
Chu XP, Xiong ZG. Chu XP, et al. Adv Exp Med Biol. 2013;961:419-31. doi: 10.1007/978-1-4614-4756-6_36. Adv Exp Med Biol. 2013. PMID: 23224900 Free PMC article. Review. - Functional communication between IP3R and STIM2 at subthreshold stimuli is a critical checkpoint for initiation of SOCE.
Ahmad M, Ong HL, Saadi H, Son GY, Shokatian Z, Terry LE, Trebak M, Yule DI, Ambudkar I. Ahmad M, et al. Proc Natl Acad Sci U S A. 2022 Jan 18;119(3):e2114928118. doi: 10.1073/pnas.2114928118. Proc Natl Acad Sci U S A. 2022. PMID: 35022238 Free PMC article. - Agonist-induced calcium entry correlates with STIM1 translocation.
Ross K, Whitaker M, Reynolds NJ. Ross K, et al. J Cell Physiol. 2007 Jun;211(3):569-76. doi: 10.1002/jcp.20993. J Cell Physiol. 2007. PMID: 17299780 Free PMC article. - Selective activation of distinct Orai channels by STIM1.
Shuttleworth TJ. Shuttleworth TJ. Cell Calcium. 2017 May;63:40-42. doi: 10.1016/j.ceca.2016.11.001. Epub 2016 Nov 4. Cell Calcium. 2017. PMID: 27847114 Free PMC article. Review. - CREB regulates the expression of type 1 inositol 1,4,5-trisphosphate receptors.
Arige V, Terry LE, Malik S, Knebel TR, Wagner Ii LE, Yule DI. Arige V, et al. J Cell Sci. 2021 Oct 15;134(20):jcs258875. doi: 10.1242/jcs.258875. Epub 2021 Oct 26. J Cell Sci. 2021. PMID: 34533188 Free PMC article.
References
- Bodding M. Activation of store-operated Ca2+ entry in RBL cells without the contribution of protein kinases. Naunyn Schmiedebergs Arch Pharmacol. 2001;363:633–638. - PubMed
- Carr DW, Stofko-Hahn RE, Fraser ID, Cone RD, Scott JD. Localization of the cAMP-dependent protein kinase to the postsynaptic densities by A-kinase anchoring proteins. Characterization of AKAP 79. J Biol Chem. 1992;267:16816–16823. - PubMed
- Coghlan VM, Perrino BA, Howard M, Langeberg LK, Hicks JB, Gallatin WM, Scott JD. Association of protein kinase A and protein phosphatase 2B with a common anchoring protein. Science. 1995;267:108–111. - PubMed
- Colledge M, Scott JD. AKAPs: from structure to function. Trends Cell Biol. 1999;9:216–221. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous