Role of insulin-induced reactive oxygen species in the insulin signaling pathway - PubMed (original) (raw)
Review
Role of insulin-induced reactive oxygen species in the insulin signaling pathway
Barry J Goldstein et al. Antioxid Redox Signal. 2005 Jul-Aug.
Abstract
Oxidants, including hydrogen peroxide (H2O2), have been recognized for years to mimic insulin action on glucose transport in adipose cells. Early studies also demonstrated the complementary finding that H2O2 was elaborated during treatment of cells with insulin, suggesting that cellular H2O2 generation was integral to insulin signaling. Recently, reactive oxygen species elicited by various hormones and growth factors have been shown to affect signal transduction pathways in various cell types. We recently reported that insulin-stimulated H2O2 modulates proximal and distal insulin signaling, at least in part through the oxidative inhibition of protein tyrosine phosphatases (PTPases) that negatively regulate the insulin action pathway. Nox4, a homologue in the family of NADPH oxidase catalytic subunits, was found to be prominently expressed in insulin-sensitive cells. By various molecular approaches, Nox4 was shown to mediate insulin-stimulated H2O2 generation and impact the insulin signaling cascade. Overexpression of Nox4 also significantly reversed the inhibition of insulin-stimulated receptor tyrosine phosphorylation by PTP1B, a widely expressed PTPase implicated in the negative regulation of insulin signaling, by inhibiting its catalytic activity. These recent studies have provided insight into Nox4 as a novel molecular link between insulin-stimulated reactive oxygen species and mechanisms involved in their modulation of insulin signal transduction.
Figures
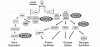
FIG. 1.
Potential sites of action for reactive oxygen species in the regulation of the insulin signaling pathway. This simplified scheme shows the major pathways of insulin signaling, involving activation of the receptor tyrosine kinase cascade leading initially to the tyrosine phosphorylation of the receptor and its cellular substrate IRS proteins and Shc (63). The steady-state level of tyrosine phosphorylation of these proteins is regulated by PTPases, a family of enzymes that constitute one of the key targets of regulation by reactive oxygen species in the cell. Additional thiol-sensitive regulatory proteins that strongly impact on the insulin action cascade include PTEN, PP2A, and MKP-1. See text for discussion and additional references. GSK3, glycogen synthase kinase 3; PDK, phospholipid-dependent kinase; PKC, protein kinase C.
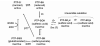
FIG. 2.
**Regulation of PTPase catalytic activity by oxidation, conjugation, and reduction of the catalytic cysteine residue.**A key feature of PTPases in the regulation of signal transduction is the modulation of their activity by alterations of the oxidation-reduction state of the enzyme catalytic center. The catalytic cysteine of the PTPases is especially reactive because of the low p_K_a of the sulfhydryl that favors a relatively ionized state of the cysteinyl hydrogen (61), and stepwise oxidation of the catalytic cysteine residue of PTPases by reactive oxygen species to more inert forms is a major source of enzymatic regulation in vivo (17, 23, 53). Oxidation of the catalytic thiol to the sulfenic (—SOH) form is reversible. Higher order oxidation to sulfinic (—SO2H) and sulfonic (—SO3H) forms can lead to irreversible PTPase inactivation. Mildly oxidized PTPases can undergo disulfide conjugation in the cell with glutathione (6). A novel sulfenyl-amide derivative of PTP1B has been shown to undergo GSH conjugation or direct biochemical reduction with DTT (62, 72). Active enzyme can be regenerated from glutathiolated enzyme by cellular GSH reductases. Whereas the sulfonic-acid derivative of the active site cysteine is felt to be irreversible, the sulfinic-acid derivative may be reduced by a novel class of recently described sulfiredoxin enzymes (7) to a conjugated form that precedes the regeneration of the active enzyme. These mechanisms of PTPase regulation are an active area of current research. See text for further discussion and references.
FIG. 3.
Insulin-stimulated production of H2O2 in 3T3-L1 adipocytes. Differentiated 3T3-L1 adipocytes were serum-starved overnight prior to stimulation with insulin as shown. Intracellular H2O2 production was detected by preloading the cells with DCF (Molecular Probes) and detecting the fluorescent signal in situ by confocal microscopy. (A) Insulin time course performed using 100 n_M_ insulin. (B) Insulin dose response performed with insulin stimulation for 5 min [modified from Mahadev et al. (47)].
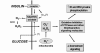
FIG. 4.
Potential mechanisms and regulation of insulin-stimulated reactive oxygen species in insulin target cells. The generation of cellular reactive oxygen species [superoxide (O2⋅−), H2O2] to insulin is coupled to a plasma membrane NADPH oxidase mechanism that we have found to involve the recently described catalytic subunit homologue, Nox4 (48). Superoxide generated by the NADPH oxidase system is potentially converted to H2O2 species can play a role in modifying the catalytic activity of thiol-dependent regulatory enzymes in the cell, which can then alter by superoxide dismutase (SOD). Both of these reactive oxygen both proximal and distal insulin action (Fig. 1). The generation of reactive oxygen species by insulin may be enhanced by high glucose conditions, which increase mitochondrial superoxide production (9) and may also activate the NADPH oxidase system. Gαi2 may also modulate the activity of the insulin-sensitive NADPH oxidase pathway (35, 38). See text for further discussion. IR, insulin receptor.
Similar articles
- The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction.
Mahadev K, Motoshima H, Wu X, Ruddy JM, Arnold RS, Cheng G, Lambeth JD, Goldstein BJ. Mahadev K, et al. Mol Cell Biol. 2004 Mar;24(5):1844-54. doi: 10.1128/MCB.24.5.1844-1854.2004. Mol Cell Biol. 2004. PMID: 14966267 Free PMC article. - Redox paradox: insulin action is facilitated by insulin-stimulated reactive oxygen species with multiple potential signaling targets.
Goldstein BJ, Mahadev K, Wu X. Goldstein BJ, et al. Diabetes. 2005 Feb;54(2):311-21. doi: 10.2337/diabetes.54.2.311. Diabetes. 2005. PMID: 15677487 Free PMC article. Review. - Modulation of insulin signal transduction by eutopic overexpression of the receptor-type protein-tyrosine phosphatase LAR.
Zhang WR, Li PM, Oswald MA, Goldstein BJ. Zhang WR, et al. Mol Endocrinol. 1996 May;10(5):575-84. doi: 10.1210/mend.10.5.8732688. Mol Endocrinol. 1996. PMID: 8732688 - Insulin-stimulated hydrogen peroxide reversibly inhibits protein-tyrosine phosphatase 1b in vivo and enhances the early insulin action cascade.
Mahadev K, Zilbering A, Zhu L, Goldstein BJ. Mahadev K, et al. J Biol Chem. 2001 Jun 15;276(24):21938-42. doi: 10.1074/jbc.C100109200. Epub 2001 Apr 10. J Biol Chem. 2001. PMID: 11297536 - Regulation of insulin receptor signaling by protein-tyrosine dephosphorylation.
Goldstein BJ. Goldstein BJ. Receptor. 1993 Spring;3(1):1-15. Receptor. 1993. PMID: 8394171 Review.
Cited by
- Photobiomodulation therapy ameliorates hyperglycemia and insulin resistance by activating cytochrome c oxidase-mediated protein kinase B in muscle.
Gong L, Zou Z, Liu L, Guo S, Xing D. Gong L, et al. Aging (Albany NY). 2021 Mar 26;13(7):10015-10033. doi: 10.18632/aging.202760. Epub 2021 Mar 26. Aging (Albany NY). 2021. PMID: 33795530 Free PMC article. - α6-Containing GABAA Receptors Are the Principal Mediators of Inhibitory Synapse Strengthening by Insulin in Cerebellar Granule Cells.
Accardi MV, Brown PM, Miraucourt LS, Orser BA, Bowie D. Accardi MV, et al. J Neurosci. 2015 Jul 1;35(26):9676-88. doi: 10.1523/JNEUROSCI.0513-15.2015. J Neurosci. 2015. PMID: 26134650 Free PMC article. - ROS signaling and redox biology in endothelial cells.
Panieri E, Santoro MM. Panieri E, et al. Cell Mol Life Sci. 2015 Sep;72(17):3281-303. doi: 10.1007/s00018-015-1928-9. Epub 2015 May 14. Cell Mol Life Sci. 2015. PMID: 25972278 Free PMC article. Review. - Hyperglycemia potentiates H(2)O(2) production in adipocytes and enhances insulin signal transduction: potential role for oxidative inhibition of thiol-sensitive protein-tyrosine phosphatases.
Wu X, Zhu L, Zilbering A, Mahadev K, Motoshima H, Yao J, Goldstein BJ. Wu X, et al. Antioxid Redox Signal. 2005 May-Jun;7(5-6):526-37. doi: 10.1089/ars.2005.7.526. Antioxid Redox Signal. 2005. PMID: 15889998 Free PMC article. - Dicholine salt of succinic acid, a neuronal insulin sensitizer, ameliorates cognitive deficits in rodent models of normal aging, chronic cerebral hypoperfusion, and beta-amyloid peptide-(25-35)-induced amnesia.
Storozheva ZI, Proshin AT, Sherstnev VV, Storozhevykh TP, Senilova YE, Persiyantseva NA, Pinelis VG, Semenova NA, Zakharova EI, Pomytkin IA. Storozheva ZI, et al. BMC Pharmacol. 2008 Jan 23;8:1. doi: 10.1186/1471-2210-8-1. BMC Pharmacol. 2008. PMID: 18215309 Free PMC article.
References
- Babior BM, Lambeth JD, Nauseef W. The neutrophil NADPH oxidase. Arch Biochem Biophys. 2002;397:342–344. - PubMed
- Bae YS, Sung JY, Kim OS, Kim YJ, Hur KC, Kazlauskas A, Rhee SG. Platelet-derived growth factor-induced H2O2 production requires the activation of phosphatidylinositol 3-kinase. J Biol Chem. 2000;275:10527–10531. - PubMed
- Barford D, Das AK, Egloff MP. The structure and mechanism of protein phosphatases—insights into catalysis and regulation. Annu Rev Biophys Biomol Struct. 1998;27:133–164. - PubMed
- Barrett WC, DeGnore JP, Keng YF, Zhang ZY, Yim MB, Chock PB. Roles of superoxide radical anion in signal transduction mediated by reversible regulation of protein-tyrosine phosphatase 1B. J Biol Chem. 1999;274:34543–34546. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous