The phagosomal transporter A couples threonine acquisition to differentiation and replication of Legionella pneumophila in macrophages - PubMed (original) (raw)
Comparative Study
. 2005 Jul 12;102(28):9924-9.
doi: 10.1073/pnas.0502767102. Epub 2005 Jul 5.
Affiliations
- PMID: 15998735
- PMCID: PMC1174991
- DOI: 10.1073/pnas.0502767102
Comparative Study
The phagosomal transporter A couples threonine acquisition to differentiation and replication of Legionella pneumophila in macrophages
John-Demian Sauer et al. Proc Natl Acad Sci U S A. 2005.
Abstract
Differentiation in response to environmental cues is integral to the success of many intracellular pathogens. By characterizing a Legionella pneumophila mutant defective for differentiation in broth and replication in macrophages, we identified a subfamily of major facilitator superfamily transporters, here named Pht (phagosomal transporter), that also is conserved in two other vacuolar pathogens, Coxiella burnetii and Francisella tularensis. Biolog phenotype microarray analysis indicated that PhtA transports threonine, an essential amino acid. Either excess threonine or threonine peptides bypass phtA function. In minimal medium, phtA mutants do not replicate; in rich broth, the bacteria prematurely differentiate to the transmissive phase, as judged by the kinetics of flaA-gfp expression, heat resistance, and sodium sensitivity. PhtA is dispensable for transmissive L. pneumophila to establish and persist within a replication vacuole but is essential for their differentiation to the replicative phase, based on phenotypic and RT-PCR analysis. Accordingly, we propose that the Pht transporter family equips transmissive L. pneumophila, C. burnetii, and F. tularensis to assess their phagosomal nutrient supply before committing to reenter the cell cycle.
Figures
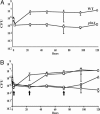
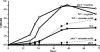
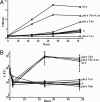
Fig. 1.
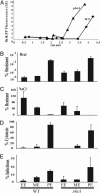
phtA mutants are defective for intracellular replication but can be rescued by complementation in trans any time after infection. (A) WT Legionella (diamonds) infect macrophages and replicate efficiently, whereas phtA mutants (squares) infect macrophages like WT, but their yield does not increase. (B) β-
d
-Thiogalactoside (IPTG) induction of the phtA locus on pMMB206::phtA (arrows) after 0 h (diamonds), 24 h (squares), or even 72 h (triangles) restores replication, whereas the yield of uninduced mutants (circles) remains constant, as judged by enumerating colony-forming units (CFU). Each experiment was performed in triplicate, and means and standard deviations are shown.
Fig. 2.
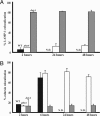
phtA mutants traffic in macrophages like WT Legionella. (A) WT bacteria (black bars) and phtA mutants (white bars) initially avoid LAMP-1-labeled compartments whereas dotA mutants (gray bars) do not. phtA mutants continue to avoid LAMP-1 endosomes throughout the infection. (B) WT bacteria (black bars) and phtA mutants (white bars) associate with calnexin by 6 h after infection, unlike dotA mutant vacuoles (gray bars). phtA mutant vacuoles remain associated with ER and isolated from the endosomal pathway for the duration of the infection. Fifty vacuoles per condition were scored for each time point, and experiments were performed in triplicate (A) or duplicate (B). Means and standard deviations are presented.
Fig. 3.
phtA mutants, which do not grow in MRM, can be fully rescued by the addition of tryptone but only partially rescued with casamino acids. WT bacteria (solid lines, filled squares) replicate slowly and to low densities in MRM, but phtA mutants (solid line, filled circles) do not. When MRM is supplemented with 5 g/liter casamino acids, WT (dotted line, filled squares) and phtA mutants (dotted line, filled circles) are only partially aided, but supplementation with 5 g/liter tryptone restores WT (solid lines, open squares) and phtA mutant (solid line, open circles) growth alike. A representative of three independent experiments performed in triplicate is shown.
Fig. 4.
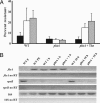
Dipeptides that contain threonine or excess free threonine bypass phtA function in broth and macrophages. (A) Defined media were supplemented with 3 mM of the indicated amino acid or peptide; then phtA mutant growth was assessed by measuring culture density (OD600). The asterisk represents the following independent samples: phtA Met, phtA, phtA Val, phtA Ala-Leu, phtA Ser, and phtA Arg from top to bottom, respectively, none of which replicated. This experiment was performed in triplicate, and a representative of three independent experiments is shown. (B) Macrophage culture media were supplemented with 3 mM peptide or amino acid as indicated. The asterisk represents the following independent samples: phtA Arg, phtA Ala-Leu, phtA Cys, phtA Ser, and phtA, from top to bottom, respectively; none of these replicated. This experiment was performed in triplicate; means and standard deviations are presented.
Fig. 5.
Intracellular phtA mutants fail to differentiate. (A) WT, phtA mutants, and phtA mutants supplemented with 3 mM threonine obtained from macrophages after 1 h (black bars) are sensitive to 100 mM NaCl. By 2 h (white bars) or 10 h (gray bars) after infection, WT and threonine-supplemented phtA mutants are NaCl-resistant, but without supplementation, phtA mutants remain sodium-sensitive. Experiments were performed in triplicate, and means and standard deviations are presented. (B) WT, phtA mutants, and phtA mutants supplemented with 3 mM threonine were lysed from host cells 1 h or 10 h postinfection; then their expression of the replicative phase rpoB gene and the transmissive phase flaA gene was analyzed by RT-PCR. As a reference for the replicative (WT EE) and transmissive (WT PE) differentiation state, RNA purified from broth cultures was analyzed; to control for genomic DNA contamination, non-reverse-transcribed RNA samples also were analyzed (no RT). 16s rRNA was analyzed as a loading control.
Fig. 6.
Premature activation of certain transmission traits by phtA mutants replicating in broth. (A) Fluorescence (AU, arbitrary units) from cultures carrying the pflaG reporter indicates that phtA mutant (triangles) express flagellin earlier than WT cells do (squares). The experiment was performed in duplicate, and a representative experiment is shown. (B_–_E) phtA mutants prematurely express heat resistance (B) and NaCl resistance (C), but not cytotoxicity (D) or infectivity (E), as judged by comparing phtA mutants and WT bacteria obtained from ME cultures (OD600 2.5). Replicative phase (OD600 1.5; EE) and transmissive phase (OD600 3.5–4.0; PE) WT and phtA mutant cultures serve as references. Each assay was conducted in triplicate, and the means and standard deviations are shown.
Fig. 7.
PhtA couples nutrient acquisition to microbial differentiation at two stages of the L. pneumophila life cycle. PhtA threonine transport triggers intracellular transmissive L. pneumophila to differentiate to the replicative form (Fig. 5). Threonine acquisition by means of PhtA also is essential for L. pneumophila to replicate in macrophages (Figs. 1 and 4). When threonine acquisition by replicating bacteria is inefficient, L. pneumophila prematurely express transmission traits (Fig. 6), perhaps due to an early accumulation of the alarmone ppGpp.
Similar articles
- Nutrient salvaging and metabolism by the intracellular pathogen Legionella pneumophila.
Fonseca MV, Swanson MS. Fonseca MV, et al. Front Cell Infect Microbiol. 2014 Feb 11;4:12. doi: 10.3389/fcimb.2014.00012. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 24575391 Free PMC article. Review. - Specificity of Legionella pneumophila and Coxiella burnetii vacuoles and versatility of Legionella pneumophila revealed by coinfection.
Sauer JD, Shannon JG, Howe D, Hayes SF, Swanson MS, Heinzen RA. Sauer JD, et al. Infect Immun. 2005 Aug;73(8):4494-504. doi: 10.1128/IAI.73.8.4494-4504.2005. Infect Immun. 2005. PMID: 16040960 Free PMC article. - The phtC-phtD locus equips Legionella pneumophila for thymidine salvage and replication in macrophages.
Fonseca MV, Sauer JD, Crepin S, Byrne B, Swanson MS. Fonseca MV, et al. Infect Immun. 2014 Feb;82(2):720-30. doi: 10.1128/IAI.01043-13. Epub 2013 Dec 2. Infect Immun. 2014. PMID: 24478086 Free PMC article. - Coxiella burnetii express type IV secretion system proteins that function similarly to components of the Legionella pneumophila Dot/Icm system.
Zamboni DS, McGrath S, Rabinovitch M, Roy CR. Zamboni DS, et al. Mol Microbiol. 2003 Aug;49(4):965-76. doi: 10.1046/j.1365-2958.2003.03626.x. Mol Microbiol. 2003. PMID: 12890021 - The role of Rab GTPases in the transport of vacuoles containing Legionella pneumophila and Coxiella burnetii.
Hardiman CA, McDonough JA, Newton HJ, Roy CR. Hardiman CA, et al. Biochem Soc Trans. 2012 Dec 1;40(6):1353-9. doi: 10.1042/BST20120167. Biochem Soc Trans. 2012. PMID: 23176480 Review.
Cited by
- Membrane vesicles shed by Legionella pneumophila inhibit fusion of phagosomes with lysosomes.
Fernandez-Moreira E, Helbig JH, Swanson MS. Fernandez-Moreira E, et al. Infect Immun. 2006 Jun;74(6):3285-95. doi: 10.1128/IAI.01382-05. Infect Immun. 2006. PMID: 16714556 Free PMC article. - IcgA is a virulence factor of Rhodococcus equi that modulates intracellular growth.
Wang X, Coulson GB, Miranda-Casoluengo AA, Miranda-Casoluengo R, Hondalus MK, Meijer WG. Wang X, et al. Infect Immun. 2014 May;82(5):1793-800. doi: 10.1128/IAI.01670-13. Epub 2014 Feb 18. Infect Immun. 2014. PMID: 24549327 Free PMC article. - Intra-Species and Inter-Kingdom Signaling of Legionella pneumophila.
Hochstrasser R, Hilbi H. Hochstrasser R, et al. Front Microbiol. 2017 Feb 3;8:79. doi: 10.3389/fmicb.2017.00079. eCollection 2017. Front Microbiol. 2017. PMID: 28217110 Free PMC article. Review.
References
- Ohta, N. & Newton, A. (1996) Trends Microbiol. 4, 326–332. - PubMed
- Kaiser, D. (1999) Trends Genet. 15, 273–277. - PubMed
- Setlow, P. (2003) Curr. Opin. Microbiol. 6, 550–556. - PubMed
- Glavin, F. L., Winn, W. C., Jr., & Craighead, J. E. (1979) Ann. Intern. Med. 90, 555–559. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources