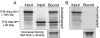Interaction between the shuttling mRNA export factor Gle1 and the nucleoporin hCG1: a conserved mechanism in the export of Hsp70 mRNA - PubMed (original) (raw)
Interaction between the shuttling mRNA export factor Gle1 and the nucleoporin hCG1: a conserved mechanism in the export of Hsp70 mRNA
Frederic Kendirgi et al. Mol Biol Cell. 2005 Sep.
Abstract
Translocation of messenger RNAs through the nuclear pore complex (NPC) requires coordinated physical interactions between stable NPC components, shuttling transport factors, and mRNA-binding proteins. In budding yeast (y) and human (h) cells, Gle1 is an essential mRNA export factor. Nucleocytoplasmic shuttling of hGle1 is required for mRNA export; however, the mechanism by which hGle1 associates with the NPC is unknown. We have previously shown that the interaction of hGle1 with the nucleoporin hNup155 is necessary but not sufficient for targeting hGle1 to NPCs. Here, we report that the unique C-terminal 43 amino acid region of the hGle1B isoform mediates binding to the C-terminal non-FG region of the nucleoporin hCG1/NPL1. Moreover, hNup155, hGle1B, and hCG1 formed a heterotrimeric complex in vitro. This suggested that these two nucleoporins were required for the NPC localization of hGle1. Using an siRNA-based approach, decreased levels of hCG1 resulted in hGle1 accumulation in cytoplasmic foci. This was coincident with inhibition of heat shock-induced production of Hsp70 protein and export of the Hsp70 mRNA in HeLa cells. Because this closely parallels the role of the hCG1 orthologue yNup42/Rip1, we speculate that hGle1-hCG1 function in the mRNA export mechanism is highly conserved.
Figures
Figure 1.
Schematic representation of structural and functional features of hGle1B and hGle1A. Amino acid (aa) residues 1-29 delineate hNup155-binding domain (Nup155BD; Rayala et al., 2004), aa 105-360 the predicted coiled-coil domain, aa 444-483 the nucleocytoplasmic shuttling domain required for hGle1-mediated mRNA export (Kendirgi et al., 2003), aa 484-559 a novel nuclear localization region (NLR) with weak nuclear import activity (Kendirgi and Wente, unpublished results), and aa 655-698 the hCG1-binding domain (hCG1BD) which is absent from hGle1A (Kendirgi et al., 2003). The regions used in Figure 3B as His-tagged fusions (hGle1B548-698 and hGle1B655-698) are also shown.
Figure 2.
hGle1B and hCG1 interact in the yeast two-hybrid assay. A schematic representation of hCG1 domain structure is shown. Amino acid (aa) residues 1-24 form a Zinc finger domain (ZF), aa 165-203 a predicted coiled-coil domain (C-C), aa 204-365 the FG-repeat domain (harboring phenylalanine (F)-glycine (G) repeats), and aa 366-423 the carboxy (C)-terminal domain. Three classes of GAD-hCG1 clones were isolated in the yeast two-hybrid screen and are highlighted in the shaded area. Interaction in the two-hybrid assay was detected by growth on media lacking leucine, uracil, histidine, and adenine (-LUHA) (for GBD-hGle1 and GBD-Snf1) or lacking leucine, uracil, histidine, and tryptophan (for GBD-hGle1A) (unpublished data). Growth was scored with (-) representing no growth and (+) representing growth. Each GAD fusion was tested for specificity by cotransformation with GBD-Snf1 and for self-activation (unpublished data).
Figure 3.
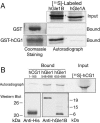
hCG1 interacts in vitro specifically with the unique hGle1B 43 aa C-terminal region. (A) Bacterially expressed/purified GST and GST-hCG1 (left) were incubated with in vitro transcribed-translated 35S-labeled FL hGle1A and hGle1B. Input and bound fractions were resolved by SDS-PAGE and detected by Coomassie staining or autoradiography. Each lane represents 20% of the input or bound fractions for Gle1. (B) In vitro transcribed-translated [35S]hCG1 was incubated with bacterially expressed and purified His-tagged hGle1548-698 and His-hGle1655-698. As a negative control, a His-fusion of hCG1 region spanning amino acids 1-165 was used. Samples were resolved by SDS-PAGE and analyzed by autoradiography or Western blotting. The Western blot with anti-His and anti-hGle1B peptide antibodies shows the recombinant protein used in the respective binding assay. Each lane represents 20% of the input or bound fractions for hCG1.
Figure 4.
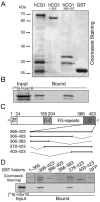
hGle1B binds the non-FG C-terminal region of hCG1. Bacterially expressed, purified GST-hCG1 fusions or GST alone were incubated with in vitro-transcribed/translated [35S]hGle1B and glutathione-Sepharose beads. Bound proteins were eluted and analyzed by SDS-PAGE followed by Coomassie staining (A) or autoradiography (B). (C) Schematic representation of the hCG1 protein/domains and the various deletions of hCG1 C-terminal domain (hCG1366-423) generated as fusions with GST in bacteria. (D) Delineating the minimal region in hCG1 capable of binding hGle1B. [35S]hGle1B was expressed in a rabbit reticulocyte lysate system and incubated with the various GST-hCG1 fusions as well as with GST-hCG11-365 and GST-GFP (negative controls). Binding of [35S]hGle1B was only detected with GST-hCG1366-423 and GST-hCG1379-423. In A, B, and C, each lane represents 20% of the respective reaction. Asterisk (*) indicates degradation products.
Figure 5.
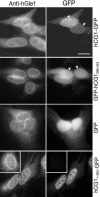
Localization of endogenous hGle1 and GFP-tagged hCG1 proteins in HeLa cells. HeLa cells were transiently transfected with plasmids expressing GFP, FL hCG1-GFP, hCG11-365-GFP, and GFP-hCG1366-423. Indirect immunofluorescence with anti-hGle1 polyclonal antibodies was used to detect endogenous hGle1. GFP and GFP-tagged proteins were detected by direct fluorescence. (arrows) highlight NE rim. Bar, 10 μm.
Figure 6.
hNup155, hCG1, and hGle1B form a heterotrimeric complex in vitro. In vitro transcribed-translated [35S]hNup155 was incubated with bacterially expressed/purified GST-hCG1 in the presence (A) or absence (B) of [35S]hGle1B. The bound proteins were isolated with glutathione-Sepharose, eluted, and separated by SDS-PAGE. Proteins were detected by Coomassie staining or autoradiography. Twenty percent of each reaction was analyzed.
Figure 7.
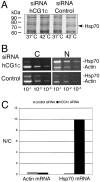
Specific hCG1C siRNA depletion of hCG1 protein levels in HeLa cells. Forty-eight hours after cotransfection with hCG1-GFP expression plasmid and 30 nM of hCG1C siRNAs or control siRNAs, HeLa cells were lysed in SDS-sample buffer and fractions separated by SDS-PAGE for Western blotting. Membranes were blotted with anti-GFP (hCG1-GFP), anti-β actin (actin), mAb414 (p62), anti-Tap (Tap), anti-Nup153 (Nup153), anti-Nup155 (Nup155), anti-Transportin 1 (Trn1), anti-Nup116 GLFG (Nup98), and anti-hGle1B peptide anti-bodies (hGle1B).
Figure 8.
siRNA depletion of hCG1 inhibits Hsp70 protein production and nuclear export of Hsp70 mRNA. (A) Assessment of Hsp70 heat-shock protein expression levels in siRNA transfected cells. Forty-eight hours after transfection with 30 nM of hCG1C siRNAs or control siRNAs, HeLa cells were heat-shocked for 2 h in the presence of [35S]methionine. Total cell protein was subsequently extracted in SDS-sample buffer and resolved by SDS-PAGE followed by autoradiography. (B) Semiquantitative analysis of actin and Hsp70 mRNA distribution in subcellular fractions of siRNA treated HeLa cells after heat shock. After treatment with siRNA pools, cells were heat-shocked at 42°C for 2 h and total RNA from nuclear and cytoplasmic fractions was isolated and reverse-transcribed with actin- and Hsp70-specific primers. Serial dilutions were made and subsaturating PCR-based amplifications were performed. The products were separated on agarose gels and stained with ethidium bromide. (N, nuclear fraction; C, cytoplasmic fraction). (C) Quantitative analysis of the representative fractionation experiment in B. Bands corresponding to Hsp70 and actin PCR products in the linear dilution range (10-2 dilution) were quantified (see Materials and Methods), and the respective nuclear/cytoplasmic ratio was plotted as a bar graph.
Figure 9.
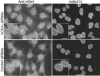
Analysis of endogenous hGle1 localization in siRNA-treated HeLa cells. Forty-eight hours after transfection with 30 nM hCG1C siRNAs or control siRNAs, cells were fixed and double indirect immunofluorescence microscopy was conducted. Endogenous hGle1 was localized using polyclonal rabbit anti-hGle1 antibodies, co-incident with NPC protein detection by monoclonal antibody 414. Ba, 10 μm.
Similar articles
- The mRNA export factor human Gle1 interacts with the nuclear pore complex protein Nup155.
Rayala HJ, Kendirgi F, Barry DM, Majerus PW, Wente SR. Rayala HJ, et al. Mol Cell Proteomics. 2004 Feb;3(2):145-55. doi: 10.1074/mcp.M300106-MCP200. Epub 2003 Nov 25. Mol Cell Proteomics. 2004. PMID: 14645504 - An essential role for hGle1 nucleocytoplasmic shuttling in mRNA export.
Kendirgi F, Barry DM, Griffis ER, Powers MA, Wente SR. Kendirgi F, et al. J Cell Biol. 2003 Mar 31;160(7):1029-40. doi: 10.1083/jcb.200211081. J Cell Biol. 2003. PMID: 12668658 Free PMC article. - Formation of Tap/NXT1 heterodimers activates Tap-dependent nuclear mRNA export by enhancing recruitment to nuclear pore complexes.
Wiegand HL, Coburn GA, Zeng Y, Kang Y, Bogerd HP, Cullen BR. Wiegand HL, et al. Mol Cell Biol. 2002 Jan;22(1):245-56. doi: 10.1128/MCB.22.1.245-256.2002. Mol Cell Biol. 2002. PMID: 11739738 Free PMC article. - A novel family of nuclear transport receptors mediates the export of messenger RNA to the cytoplasm.
Izaurralde E. Izaurralde E. Eur J Cell Biol. 2002 Nov;81(11):577-84. doi: 10.1078/0171-9335-00273. Eur J Cell Biol. 2002. PMID: 12498157 Review. - Insights into mRNA export-linked molecular mechanisms of human disease through a Gle1 structure-function analysis.
Folkmann AW, Dawson TR, Wente SR. Folkmann AW, et al. Adv Biol Regul. 2014 Jan;54:74-91. doi: 10.1016/j.jbior.2013.10.002. Epub 2013 Nov 13. Adv Biol Regul. 2014. PMID: 24275432 Free PMC article. Review.
Cited by
- mRNA nuclear export and human disease.
Hurt JA, Silver PA. Hurt JA, et al. Dis Model Mech. 2008 Sep-Oct;1(2-3):103-8. doi: 10.1242/dmm.000745. Dis Model Mech. 2008. PMID: 19048072 Free PMC article. - Distinct roles of nuclear basket proteins in directing the passage of mRNA through the nuclear pore.
Li Y, Aksenova V, Tingey M, Yu J, Ma P, Arnaoutov A, Chen S, Dasso M, Yang W. Li Y, et al. Proc Natl Acad Sci U S A. 2021 Sep 14;118(37):e2015621118. doi: 10.1073/pnas.2015621118. Proc Natl Acad Sci U S A. 2021. PMID: 34504007 Free PMC article. - Nup42 and IP6 coordinate Gle1 stimulation of Dbp5/DDX19B for mRNA export in yeast and human cells.
Adams RL, Mason AC, Glass L, Aditi, Wente SR. Adams RL, et al. Traffic. 2017 Dec;18(12):776-790. doi: 10.1111/tra.12526. Epub 2017 Oct 16. Traffic. 2017. PMID: 28869701 Free PMC article. - Survival beyond the perinatal period expands the phenotypes caused by mutations in GLE1.
Said E, Chong JX, Hempel M, Denecke J, Soler P, Strom T, Nickerson DA, Kubisch C; University of Washington Center for Mendelian Genomics; Bamshad MJ, Lessel D. Said E, et al. Am J Med Genet A. 2017 Nov;173(11):3098-3103. doi: 10.1002/ajmg.a.38406. Epub 2017 Sep 8. Am J Med Genet A. 2017. PMID: 28884921 Free PMC article. - Interactome Mapping Reveals the Evolutionary History of the Nuclear Pore Complex.
Obado SO, Brillantes M, Uryu K, Zhang W, Ketaren NE, Chait BT, Field MC, Rout MP. Obado SO, et al. PLoS Biol. 2016 Feb 18;14(2):e1002365. doi: 10.1371/journal.pbio.1002365. eCollection 2016 Feb. PLoS Biol. 2016. PMID: 26891179 Free PMC article.
References
- Bednenko, J., Cingolani, G., and Gerace, L. (2003). Nucleocytoplasmic transport: navigating the channel. Traffic 4, 127-135. - PubMed
- Blevins, M. B., Smith, A. M., Phillips, E. M., and Powers, M. A. (2003). Complex formation among the RNA export proteins Nup98, Rae1/Gle2 and TAP. J. Biol. Chem. 278, 20979-20988. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GM51219/GM/NIGMS NIH HHS/United States
- GM072272/GM/NIGMS NIH HHS/United States
- F32 GM072272/GM/NIGMS NIH HHS/United States
- R01 GM051219/GM/NIGMS NIH HHS/United States
- R37 GM051219/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous