Reducing cerebral microvascular amyloid-beta protein deposition diminishes regional neuroinflammation in vasculotropic mutant amyloid precursor protein transgenic mice - PubMed (original) (raw)
Reducing cerebral microvascular amyloid-beta protein deposition diminishes regional neuroinflammation in vasculotropic mutant amyloid precursor protein transgenic mice
Jianting Miao et al. J Neurosci. 2005.
Abstract
Cerebral microvascular amyloid-beta (Abeta) protein deposition is emerging as an important contributory factor to neuroinflammation and dementia in Alzheimer's disease and related familial cerebral amyloid angiopathy disorders. In particular, cerebral microvascular amyloid deposition, but not parenchymal amyloid, is more often correlated with dementia. Recently, we generated transgenic mice (Tg-SwDI) expressing the vasculotropic Dutch (E693Q)/Iowa (D694N) mutant human Abeta precursor protein in brain that accumulate abundant cerebral microvascular fibrillar amyloid deposits. In the present study, our aim was to assess how the presence or absence of fibrillar Abeta deposition in the cerebral microvasculature affects neuroinflammation in Tg-SwDI mice. Using Tg-SwDI mice bred onto an apolipoprotein E gene knock-out background, we found a strong reduction of fibrillar cerebral microvascular Abeta deposition, which was accompanied by a sharp decrease in microvascular-associated neuroinflammatory cells and interleukin-1beta levels. Quantitative immunochemical measurements showed that this reduction of the neuroinflammation occurred in the absence of lowering the levels of total Abeta40/Abeta42 or soluble Abeta oligomers in brain. These findings suggest that specifically reducing cerebral microvascular fibrillar Abeta deposition, in the absence of lowering either the total amount of Abeta or soluble Abeta oligomers in brain, may be sufficient to ameliorate microvascular amyloid-associated neuroinflammation.
Figures
Figure 1.
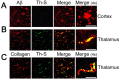
Tg-SwDI mice develop fibrillar Aβ deposits exclusively in the cerebral microvasculature. A, Abundant Aβ immunostaining, but lack of thioflavin-S (Th-S) amyloid staining, of diffuse parenchymal deposits in the neocortex of Tg-SwDI mice. B, Colocalization of Aβ immunostaining and thioflavin-S amyloid staining in the thalamus of Tg-SwDI mice is predominantly localized in microvasculature structures. C, Colocalization of vascular collagen IV immunostaining and thioflavin-S amyloid staining in the thalamus of Tg-SwDI mice. Scale bars, 50 μm.
Figure 2.
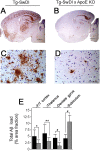
Lack of endogenous mouse apoE lowers cerebral Aβ deposition in Tg-SwDI mice. A-D, Deposition of Aβ in forebrain (A, B) and frontotemporal cortex (C, D) in Tg-SwDI in the absence or presence, respectively, of endogenous mouse apoE. Scale bars: A, B, 1 mm; C, D, 50 μm, respectively. E, Quantitative image analysis of regional Aβ deposition in Tg-SwDI mice in the presence (black bars) or absence (gray bars) of endogenous mouse apoE. Data shown are mean ± SD (_n_=10). *p < 0.01; **p < 0.02; #p < 0.001. F/T, Frontotemporal; KO, knock-out.
Figure 3.
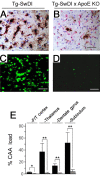
Lack of endogenous mouse apoE eliminates cerebral microvascular Aβ deposition in Tg-SwDI mice. A-D, Fibrillar microvascular amyloid deposition in Tg-SwDI mouse thalamus revealed by immunostaining for Aβ (brown) and collagen type IV (red; A, B) and thioflavin-S fluorescence staining (C, D) in the presence (A, C) or absence (B, D) of endogenous mouse apoE. Scale bars, 50 μm. E, Quantitative stereological estimation of microvascular amyloid deposition in the presence (black bars) or absence (gray bars) of endogenous apoE. Data shown are mean ± SD (n = 10). *p < 0.01; **p < 0.0001. F/T, Frontotemporal; KO, knock-out.
Figure 4.
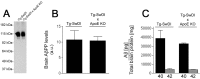
Effects of endogenous mouse apoE on cerebral human AβPP and Aβ levels in Tg-SwDI mice. A, Immunoblot analysis of transgene-encoded human AβPP levels in mouse forebrain extracts. B, Quantitation of human AβPP immunoblots (n = 4) of mouse forebrain extracts. C, ELISA measurements of total Aβ40 (black bars) and Aβ42 (gray bars) in mouse forebrain tissue. ELISA data shown are mean ± SD (n = 12-16). a.u., Arbitrary units; KO, knock-out.
Figure 5.
Effects of endogenous mouse apoE on cerebral soluble and insoluble Aβ levels in Tg-SwDI mice. A, Ratio of total insoluble Aβ/soluble Aβ. B, Representative dot-blot analysis of Aβ oligomers in soluble mouse forebrain extracts. C, Quantitation of soluble Aβ oligomers from dot blots of Tg-SwDI and _Tg-SwDI/apoE_-/- mice. Data shown are mean ± SD (n = 5). *p < 0.001. KO, Knock-out.
Figure 6.
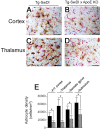
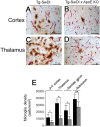
Reduced cerebral microvascular amyloid decreases reactive astrocytes in Tg-SwDI mice. A-D, Microvascular-associated reactive astrocytes revealed by GFAP-positive immunostaining (brown) and collagen type IV (red) in Tg-SwDI mouse frontotemporal cortex (A, B) or thalamus (C, D) in the presence (A, C) or absence (B, D) of endogenous mouse apoE. Scale bars, 50 μm. E, Quantitative stereological estimation of reactive astrocyte densities in brain regions of Tg-SwDI mice in the presence (black bars) or absence (gray bars) of endogenous mouse apoE. Data shown are mean ± SD (n = 10). *p < 0.001. F/T, Frontotemporal; KO, knock-out.
Figure 7.
Reduced cerebral microvascular amyloid decreases activated microglia in Tg-SwDI mice. A-D, Microvascular-associated activated microglia revealed by 5D4-positive immunostaining (brown) and collagen type IV (red) in Tg-SwDI mouse frontotemporal cortex (A, B) or thalamus (C, D) in the presence (A, C) or absence (B, D) of endogenous mouse apoE. Scale bars, 50 μm. E, Quantitative stereological estimation of activated microglial densities in brain regions of Tg-SwDI mice in the presence (black bars) or absence (gray bars) of endogenous mouse apoE. Data shown are mean ± SD (n = 10). *p < 0.0001; **p < 0.01. F/T, Frontotemporal; KO, knock-out.
Figure 8.
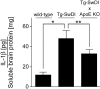
Reduced cerebral microvascular amyloid lowers the elevated IL-1β levels found in Tg-SwDI mice. The levels of IL-1β were measured in soluble forebrain extracts of 12-month-old wild-type, Tg-SwDI/apoE+/+, and _Tg-SwDI/apoE_-/- mice by ELISA analysis. Data shown are mean ± _SD (_n = _5). *_p < _0.0002; **_p < 0.01. KO, Knock-out.
Similar articles
- Cerebral microvascular amyloid beta protein deposition induces vascular degeneration and neuroinflammation in transgenic mice expressing human vasculotropic mutant amyloid beta precursor protein.
Miao J, Xu F, Davis J, Otte-Höller I, Verbeek MM, Van Nostrand WE. Miao J, et al. Am J Pathol. 2005 Aug;167(2):505-15. doi: 10.1016/s0002-9440(10)62993-8. Am J Pathol. 2005. PMID: 16049335 Free PMC article. - Minocycline reduces microglial activation and improves behavioral deficits in a transgenic model of cerebral microvascular amyloid.
Fan R, Xu F, Previti ML, Davis J, Grande AM, Robinson JK, Van Nostrand WE. Fan R, et al. J Neurosci. 2007 Mar 21;27(12):3057-63. doi: 10.1523/JNEUROSCI.4371-06.2007. J Neurosci. 2007. PMID: 17376966 Free PMC article. - Early-onset subicular microvascular amyloid and neuroinflammation correlate with behavioral deficits in vasculotropic mutant amyloid beta-protein precursor transgenic mice.
Xu F, Grande AM, Robinson JK, Previti ML, Vasek M, Davis J, Van Nostrand WE. Xu F, et al. Neuroscience. 2007 Apr 25;146(1):98-107. doi: 10.1016/j.neuroscience.2007.01.043. Epub 2007 Feb 28. Neuroscience. 2007. PMID: 17331655 Free PMC article. - Tg-SwDI transgenic mice: A suitable model for Alzheimer's disease and cerebral amyloid angiopathy basic research and preclinical studies.
Rodriguez-Lopez A, Esteban D, Domínguez-Romero AN, Gevorkian G. Rodriguez-Lopez A, et al. Exp Neurol. 2025 May;387:115189. doi: 10.1016/j.expneurol.2025.115189. Epub 2025 Feb 18. Exp Neurol. 2025. PMID: 39978567 Review. - Pathogenic effects of cerebral amyloid angiopathy mutations in the amyloid beta-protein precursor.
Van Nostrand WE, Melchor JP, Romanov G, Zeigler K, Davis J. Van Nostrand WE, et al. Ann N Y Acad Sci. 2002 Nov;977:258-65. doi: 10.1111/j.1749-6632.2002.tb04824.x. Ann N Y Acad Sci. 2002. PMID: 12480759 Review.
Cited by
- Neuronal or glial expression of human apolipoprotein e4 affects parenchymal and vascular amyloid pathology differentially in different brain regions of double- and triple-transgenic mice.
Van Dooren T, Muyllaert D, Borghgraef P, Cresens A, Devijver H, Van der Auwera I, Wera S, Dewachter I, Van Leuven F. Van Dooren T, et al. Am J Pathol. 2006 Jan;168(1):245-60. doi: 10.2353/ajpath.2006.050752. Am J Pathol. 2006. PMID: 16400027 Free PMC article. - Insights into Cerebral Amyloid Angiopathy Type 1 and Type 2 from Comparisons of the Fibrillar Assembly and Stability of the Aβ40-Iowa and Aβ40-Dutch Peptides.
Rajpoot J, Crooks EJ, Irizarry BA, Amundson A, Van Nostrand WE, Smith SO. Rajpoot J, et al. Biochemistry. 2022 Jun 21;61(12):1181-1198. doi: 10.1021/acs.biochem.1c00781. Epub 2022 Jun 6. Biochemistry. 2022. PMID: 35666749 Free PMC article. - Reduction of orexin-expressing neurons and a unique sleep phenotype in the Tg-SwDI mouse model of Alzheimer's disease.
Wu Y, Bhat NR, Liu M. Wu Y, et al. Front Aging Neurosci. 2025 Feb 4;17:1529769. doi: 10.3389/fnagi.2025.1529769. eCollection 2025. Front Aging Neurosci. 2025. PMID: 39968126 Free PMC article. - Eradication of Helicobacter pylori Is Associated with the Progression of Dementia: A Population-Based Study.
Chang YP, Chiu GF, Kuo FC, Lai CL, Yang YH, Hu HM, Chang PY, Chen CY, Wu DC, Yu FJ. Chang YP, et al. Gastroenterol Res Pract. 2013;2013:175729. doi: 10.1155/2013/175729. Epub 2013 Nov 25. Gastroenterol Res Pract. 2013. PMID: 24371435 Free PMC article. - ACE overexpression in myelomonocytic cells: effect on a mouse model of Alzheimer's disease.
Koronyo-Hamaoui M, Shah K, Koronyo Y, Bernstein E, Giani JF, Janjulia T, Black KL, Shi PD, Gonzalez-Villalobos RA, Fuchs S, Shen XZ, Bernstein KE. Koronyo-Hamaoui M, et al. Curr Hypertens Rep. 2014 Jul;16(7):444. doi: 10.1007/s11906-014-0444-x. Curr Hypertens Rep. 2014. PMID: 24792094 Free PMC article. Review.
References
- Ashford JW (2004) APOE genotype effects on Alzheimer's disease onset and epidemiology. J Mol Neurosci 23: 157-165. - PubMed
- Atterns J, Jellinger KA (2004) Only cerebral capillary amyloid angiopathy correlates with Alzheimer pathology—a pilot study. Acta Neuropathol 107: 83-90. - PubMed
- Bailey TL, Rivara CB, Rocher AB, Hof PR (2004) The nature and effects of cortical microvascular pathology in aging and Alzheimer's disease. Neurol Res 26: 573-578. - PubMed
- Bales KR, Verina T, Cummins DJ, Du Y, Dodel RC, Saura J, Fishman CE, DeLong CA, Piccardo P, Petegnief V, Ghetti B, Paul SM (1999) Apolipoprotein E is essential for amyloid deposition in the APPV717F transgenic mouse model of Alzheimer's disease. Proc Natl Acad Sci USA 96: 15233-15238. - PMC - PubMed
- Chalmers K, Wilcock GK, Love S (2003) APOEϵ4 influences the pathological phenotype of Alzheimer's disease by favouring cerebrovascular over parenchymal accumulation of Aβ protein. Neuropathol Appl Neurobiol 29: 231-238. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous