Bone marrow plasticity revisited: protection or differentiation in the kidney tubule? - PubMed (original) (raw)
Review
Bone marrow plasticity revisited: protection or differentiation in the kidney tubule?
Diane Krause et al. J Clin Invest. 2005 Jul.
Abstract
Epithelial organs such as the intestine and skin have a relatively high rate of cell loss and thus require a reservoir of stem cells capable of both replacing the lost epithelia and maintaining the reservoir. Whether the kidney has such a stem cell niche has been a subject of great interest; the majority of data suggest that replacement of renal epithelial cells occurs via dedifferentiation and proliferation of existing tubular cells, while some studies demonstrate the presence of potential tubular stem cells in the renal interstitium. However, recent reports have suggested that the bone marrow may also be a source of stem cells for tubule turnover and/or repair. In this issue of the JCI, 2 groups explore the role of endogenous cells versus bone marrow-derived cells in mediating tubule repair. Duffield and colleagues demonstrate that bone marrow does contain cells capable of protecting the kidney from ischemic injury, but found that these cells do not act by direct incorporation into the repaired tubular segments. In contrast, Lin and coworkers found that some bone marrow-derived cells do appear to incorporate into the injured tubule as epithelial cells (see the related article beginning on page 1756). Importantly, both groups conclude that the majority of tubule repair occurs via proliferation of endogenous renal cells rather than incorporation of bone marrow-derived cells.
Figures
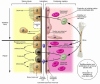
Figure 1
Potential roles of bone marrow–derived cells in renal protection/repair. After ischemic injury, the brush border of proximal tubular cells is rapidly lost, followed by either dedifferentiation and proliferation of these cells or cell detachment and death due to necrosis or apoptosis. The observation that the initial decline in renal function following ischemic injury is markedly diminished in animals receiving large numbers of MSCs suggests that these cells act to prevent the acute phase of the injury either by directly inhibiting cell death and/or by preventing inflammatory cell influx (I). Once injury has occurred, repair of the tubule requires the dedifferentiation, migration, and proliferation of the surviving tubular cells. In addition, it has been proposed that resident renal stem cells participate in the reparative phase by migrating into the tubule and differentiating into epithelial cells (14). MSCs may direct this phase of tubule repair by secreting a factor (or factors) that promotes tubular cell dedifferentiation and proliferation or by stimulating the influx of resident stem cells (II). A second bone marrow cell type, possibly HSCs, appears to serve as a reservoir of endothelial progenitor cells, which potentially promotes endothelial repair and increases renal medullary blood flow (III). Finally, the possibility remains that bone marrow–derived cells can, very rarely, undergo differentiation into or fuse with existing tubular cells to directly participate in tubule repair. The infrequent occurrence of these last 2 processes suggests that they are not critical for functional recovery from acute ischemic renal injury.
Comment on
- Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells.
Duffield JS, Park KM, Hsiao LL, Kelley VR, Scadden DT, Ichimura T, Bonventre JV. Duffield JS, et al. J Clin Invest. 2005 Jul;115(7):1743-55. doi: 10.1172/JCI22593. Epub 2005 Jun 2. J Clin Invest. 2005. PMID: 16007251 Free PMC article.
Similar articles
- Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells.
Duffield JS, Park KM, Hsiao LL, Kelley VR, Scadden DT, Ichimura T, Bonventre JV. Duffield JS, et al. J Clin Invest. 2005 Jul;115(7):1743-55. doi: 10.1172/JCI22593. Epub 2005 Jun 2. J Clin Invest. 2005. PMID: 16007251 Free PMC article. - Kidney tubular epithelium is restored without replacement with bone marrow-derived cells during repair after ischemic injury.
Duffield JS, Bonventre JV. Duffield JS, et al. Kidney Int. 2005 Nov;68(5):1956-61. doi: 10.1111/j.1523-1755.2005.00629.x. Kidney Int. 2005. PMID: 16221175 Free PMC article. Review. - Rare incorporation of bone marrow-derived cells into kidney after folic acid-induced injury.
Szczypka MS, Westover AJ, Clouthier SG, Ferrara JL, Humes HD. Szczypka MS, et al. Stem Cells. 2005;23(1):44-54. doi: 10.1634/stemcells.2004-0111. Stem Cells. 2005. PMID: 15625121 - Bone marrow contributes to renal parenchymal turnover and regeneration.
Poulsom R, Forbes SJ, Hodivala-Dilke K, Ryan E, Wyles S, Navaratnarasah S, Jeffery R, Hunt T, Alison M, Cook T, Pusey C, Wright NA. Poulsom R, et al. J Pathol. 2001 Sep;195(2):229-35. doi: 10.1002/path.976. J Pathol. 2001. PMID: 11592103 - Stem cells in kidney regeneration following acute renal injury.
Lin F. Lin F. Pediatr Res. 2006 Apr;59(4 Pt 2):74R-8R. doi: 10.1203/01.pdr.0000205156.85990.12. Pediatr Res. 2006. PMID: 16549552 Review.
Cited by
- De novo kidney regeneration with stem cells.
Yokote S, Yamanaka S, Yokoo T. Yokote S, et al. J Biomed Biotechnol. 2012;2012:453519. doi: 10.1155/2012/453519. Epub 2012 Nov 26. J Biomed Biotechnol. 2012. PMID: 23251079 Free PMC article. Review. - Bone marrow-derived endothelial progenitor cells and endothelial cells may contribute to endothelial repair in the kidney immediately after ischemia-reperfusion.
Kwon O, Miller S, Li N, Khan A, Kadry Z, Uemura T. Kwon O, et al. J Histochem Cytochem. 2010 Aug;58(8):687-94. doi: 10.1369/jhc.2010.956011. Epub 2010 Mar 30. J Histochem Cytochem. 2010. PMID: 20354148 Free PMC article. - Mesenchymal stem cells as a therapeutic approach to glomerular diseases: benefits and risks.
Kunter U, Rong S, Moeller MJ, Floege J. Kunter U, et al. Kidney Int Suppl (2011). 2011 Sep;1(3):68-73. doi: 10.1038/kisup.2011.16. Kidney Int Suppl (2011). 2011. PMID: 25018904 Free PMC article. Review. - Evidence of In Vitro Preservation of Human Nephrogenesis at the Single-Cell Level.
Pode-Shakked N, Gershon R, Tam G, Omer D, Gnatek Y, Kanter I, Oriel S, Katz G, Harari-Steinberg O, Kalisky T, Dekel B. Pode-Shakked N, et al. Stem Cell Reports. 2017 Jul 11;9(1):279-291. doi: 10.1016/j.stemcr.2017.04.026. Epub 2017 May 25. Stem Cell Reports. 2017. PMID: 28552604 Free PMC article. - Advances in the Knowledge about Kidney Decellularization and Repopulation.
Destefani AC, Sirtoli GM, Nogueira BV. Destefani AC, et al. Front Bioeng Biotechnol. 2017 Jun 1;5:34. doi: 10.3389/fbioe.2017.00034. eCollection 2017. Front Bioeng Biotechnol. 2017. PMID: 28620603 Free PMC article. Review.
References
- Poulsom R, et al. Bone marrow contributes to renal parenchymal turnover and regeneration. J. Pathol. 2001;195:229–235. - PubMed
- Gupta S, Verfaillie C, Chmielewski D, Kim Y, Rosenberg ME. A role for extrarenal cells in the regeneration following acute renal failure. Kidney Int. 2002;62:1285–1290. - PubMed
- Lin F, et al. Hematopoietic stem cells contribute to the regeneration of renal tubules after renal ischemia-reperfusion injury in mice. J. Am. Soc. Nephrol. 2003;14:1188–1199. - PubMed
- Lagasse E, et al. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat. Med. 2000;6:1229–1234. - PubMed