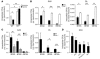HIF-1alpha expression regulates the bactericidal capacity of phagocytes - PubMed (original) (raw)
HIF-1alpha expression regulates the bactericidal capacity of phagocytes
Carole Peyssonnaux et al. J Clin Invest. 2005 Jul.
Abstract
Hypoxia is a characteristic feature of the tissue microenvironment during bacterial infection. Here we report on our use of conditional gene targeting to examine the contribution of hypoxia-inducible factor 1, alpha subunit (HIF-1alpha) to myeloid cell innate immune function. HIF-1alpha was induced by bacterial infection, even under normoxia, and regulated the production of key immune effector molecules, including granule proteases, antimicrobial peptides, nitric oxide, and TNF-alpha. Mice lacking HIF-1alpha in their myeloid cell lineage showed decreased bactericidal activity and failed to restrict systemic spread of infection from an initial tissue focus. Conversely, activation of the HIF-1alpha pathway through deletion of von Hippel-Lindau tumor-suppressor protein or pharmacologic inducers supported myeloid cell production of defense factors and improved bactericidal capacity. HIF-1alpha control of myeloid cell activity in infected tissues could represent a novel therapeutic target for enhancing host defense.
Figures
Figure 1
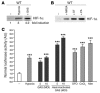
Bacteria increase HIF-1α protein expression and stimulate HIF-1α transcriptional activity. (A and B) Macrophages were incubated under hypoxia (0.1%) or with GAS, MRSA, S. typhimurium (ST), or P. aeruginosa (PA) at an MOI equal to 5–10 under normoxic conditions for 4 hours. Expression of HIF-1α was normalized to β-actin levels and quantified with ImageQuantTL software (Amersham Biosciences). (C) HRE-luciferase BM-derived macrophages were incubated either with GAS or heat-inactivated GAS at an MOI equal to 5–10 under hypoxia (1%) or with the addition of mimosine (800 μM), desferrioxamine mesylate (150 μM), or CoCl2 (150 μM) for 18 hours. Statistical analyses were performed using unpaired Student’s t test. **P < 0.01; ***P < 0.001.
Figure 2
HIF-1α regulates bactericidal activity of myeloid cells. (A) Intracellular killing of GAS by WT, HIF-1α–null, or vHL-null macrophages. BM-derived macrophages were inoculated with GAS at an MOI equal to 2.5 and cultured under normoxic (white bars) or hypoxic (0.1%; black bars) conditions for 1 hour after antibiotic treatment. Statistical analyses were performed using unpaired Student’s t test. *P < 0.05; **P < 0.01. (B) Loss of HIF-1α in macrophages decreases intracellular killing of GAS and of P. aeruginosa. WT (black bars) or HIF-1α–null (white bars) BM-derived macrophages were incubated with bacteria for 1 hour before antibiotics were added. Intracellular killing was analyzed by determination of viable CFUs in macrophage lysates at the specified time points after bacterial uptake. Experiments were performed in triplicate. SEM is displayed. Experiment shown is representative of 3 repeated studies. (C) Loss of vHL in BM-derived macrophages increases intracellular killing of GAS and of P. aeruginosa. Experiments were performed in triplicate and are representative of 3 repeated studies. SEM is displayed. (D) Pharmacologic agonists of HIF-1α increase myeloid cell bactericidal activity. Preincubation (5 hours) with desferrioxamine mesylate (DFO), CoCl2, OH-pyridone, or Mim increased the intracellular killing capacity of WT macrophages against GAS. ***P < 0.001.
Figure 3
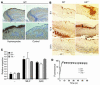
HIF-1α deletion renders mice more susceptible to GAS infection. (A) Area of necrotic ulcer and (B) loss of weight in individual WT (squares) and HIF-1α myeloid–null mice (triangles) 4 days after infection with GAS. (C) Representative appearance of GAS-induced necrotic skin ulcers in WT and HIF-1α myeloid–null mice. A total of 11 mice in each group were tested in 3 paired experiments. (D) Bacterial counts in the blood, spleen, and skin of WT and HIF-1α myeloid–null mice infected with GAS. The fold difference in quantitive GAS culture between WT and HIF-1α–null animals is annotated. Statistical analyses were performed using unpaired Student’s t test. *P < 0.05; **P < 0.01.
Figure 4
HIF-1α is not critical for neutrophil endothelial transcytosis or oxidative burst function. (A) Hypoxia is present in lesions generated by GAS infection. Immunostaining for hypoxic markers in WT mouse skin upon GAS infection. Magnification, ×100 (top); ×200 (bottom). The control corresponds to the omission of primary antibody. (B) Similar numbers of neutrophils in WT and HIF-1α–null mouse skin tissue observed by immunostaining at 6, 12, and 24 hours after infection. Magnification, ×100. (C) Migratory capacity of activated neutrophils across endothelium is not affected by the deletion of HIF-1α. Count of neutrophils transcytosing pulmonary endothelial monolayer toward GAS or fMLP stimulus is shown. (D) HIF-1α activity does not affect oxidative burst capacity. Flow cytometry of leukocytes derived from WT (squares), HIF-1α–null (triangles) and vHL-null (inverted triangles) mice. Oxidative burst capacity as measured by fluorescence before (0 seconds) and after the addition of a reagent designed to stimulate leukocyte phagocytic and oxidative activity as described in Methods. Data are representative of the results obtained for 4 individuals per genotype.
Figure 5
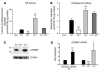
Production of granule proteases and of murine CRAMP is regulated by HIF-1α. NE (A) and cathepsin G (B) activity in WT, HIF-1α–null, vHL-null and in a mix of WT and HIF–/– blood leukocytes. (C) Neutrophils were processed for immunoblotting with anti-CRAMP antibody (upper panels) or anti–β-actin antibody (lower panels). (D) HIF-1α regulates CRAMP at the mRNA level. Neutrophils were cultured under normoxic or hypoxic (0.1%) conditions. Total neutrophil RNA was extracted and mRNA polyA+ isolated by an Oligotex mRNA spin-column protocol (QIAGEN). WT neutrophils were arbitrarily set to 1 unit following normalization to β-actin RNA levels. Statistical analyses were performed using unpaired Student’s t test. *P < 0.05; **P < 0.01; ***P < 0.001.
Figure 6
HIF-1α and vHL regulate NO production. (A) Total RNA from WT, HIF-1α–/–, and vHL–/– bone marrow–derived macrophages infected with GAS isolated 3 hours after antibiotic treatment. iNOS mRNA was quantified by RT-PCR. WT, nonstimulated macrophages were arbitrarily set to 1 unit following normalization to ribosomal RNA levels. (B) NO production under GAS stimulation ± 1.5 mM AG (1-amino-2-hydroxyguanidine, p-toluenesulfate; Calbiochem). BM-derived macrophages were cultured for 20 hours, conditioned supernatant collected, and NO protein levels measured by the Griess assay. (C) Mim enhances iNOS expression of WT macrophages stimulated by GAS. Total RNA from WT and HIF-1α–null BM-derived macrophages infected with GAS ± Mim isolated 3 hours after antibiotic treatment. iNOS mRNA was quantified by RT-PCR. WT, noninfected macrophages were arbitrarily set to 1 unit following normalization to ribosomal RNA levels. Statistical analyses performed by unpaired Student’s t test. **P < 0.01; ***P < 0.001. (D) Inhibition of iNOS by AG blunts observed differences between HIF-1α–null and WT microbicidal activity. (E) Inhibition of iNOS prevents GAS-induced HIF-1α expression. Expression of HIF-1α is normalized to β-actin levels.
Figure 7
HIF-1α and vHL regulate TNF-α production. (A) Total RNA from WT, HIF-1α–/–, and vHL–/– bone marrow–derived macrophages infected with GAS were isolated 3 hours after antibiotic treatment. TNF-α mRNA was quantified by RT-PCR. WT, nonstimulated macrophages were arbitrarily set to 1 unit following normalization to ribosomal RNA levels. (B) Inhibition of iNOS decreases TNF-α production. BM-derived macrophages were cultured for 1 hour after antibiotic treatment. Conditioned supernatant was harvested and TNF-α protein analyzed by ELISA (eBiosciences). Statistical analyses were performed using unpaired Student’s t test. ***P < 0.001.
Figure 8
Model for the role of HIF-1α in myeloid cell innate immune function. Bactericidal mechanisms can be maintained in an “off” state while myeloid cells circulate in the oxygen-rich bloodstream. Transendothelial migration toward an infectious focus occurs in a HIF-1α independent fashion, but upon diapedesis, specific bactericidal mechanisms are activated through HIF-1α induction in response to the declining oxygen gradient. Further potent stimulation of the HIF-1α transcriptional pathway is provided after direct encounter with the infecting bacterial pathogen. HIF-1α regulates the generation of critical molecular effectors of immune defense, including granule proteases, antimicrobial peptides, and TNF-α. HIF-1α also stimulates the production of NO, which not only acts as an antimicrobial agent and inflammatory mediator but further amplifies myeloid cell bactericidal activity via HIF-1α stabilization.
Comment in
- HIF-1alpha: a master regulator of innate host defenses?
Zarember KA, Malech HL. Zarember KA, et al. J Clin Invest. 2005 Jul;115(7):1702-4. doi: 10.1172/JCI25740. J Clin Invest. 2005. PMID: 16007247 Free PMC article. Review.
Similar articles
- HIF-1alpha is essential for myeloid cell-mediated inflammation.
Cramer T, Yamanishi Y, Clausen BE, Förster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, Firestein GS, Gerber HP, Ferrara N, Johnson RS. Cramer T, et al. Cell. 2003 Mar 7;112(5):645-57. doi: 10.1016/s0092-8674(03)00154-5. Cell. 2003. PMID: 12628185 Free PMC article. - Critical role of HIF-1alpha in keratinocyte defense against bacterial infection.
Peyssonnaux C, Boutin AT, Zinkernagel AS, Datta V, Nizet V, Johnson RS. Peyssonnaux C, et al. J Invest Dermatol. 2008 Aug;128(8):1964-8. doi: 10.1038/jid.2008.27. Epub 2008 Mar 6. J Invest Dermatol. 2008. PMID: 18323789 - HIF-1alpha: a master regulator of innate host defenses?
Zarember KA, Malech HL. Zarember KA, et al. J Clin Invest. 2005 Jul;115(7):1702-4. doi: 10.1172/JCI25740. J Clin Invest. 2005. PMID: 16007247 Free PMC article. Review. - Hypoxia-inducible factor (HIF-1)alpha: its protein stability and biological functions.
Lee JW, Bae SH, Jeong JW, Kim SH, Kim KW. Lee JW, et al. Exp Mol Med. 2004 Feb 29;36(1):1-12. doi: 10.1038/emm.2004.1. Exp Mol Med. 2004. PMID: 15031665 Review.
Cited by
- β2-integrins control HIF1α activation in human neutrophils.
Kling L, Eulenberg-Gustavus C, Jerke U, Rousselle A, Eckardt KU, Schreiber A, Kettritz R. Kling L, et al. Front Immunol. 2024 Oct 14;15:1406967. doi: 10.3389/fimmu.2024.1406967. eCollection 2024. Front Immunol. 2024. PMID: 39469705 Free PMC article. - A myeloid hypoxia-inducible factor 1α-Krüppel-like factor 2 pathway regulates gram-positive endotoxin-mediated sepsis.
Mahabeleshwar GH, Qureshi MA, Takami Y, Sharma N, Lingrel JB, Jain MK. Mahabeleshwar GH, et al. J Biol Chem. 2012 Jan 6;287(2):1448-57. doi: 10.1074/jbc.M111.312702. Epub 2011 Nov 22. J Biol Chem. 2012. PMID: 22110137 Free PMC article. - The dendritic cell response to classic, emerging, and homeostatic danger signals. Implications for autoimmunity.
Gallo PM, Gallucci S. Gallo PM, et al. Front Immunol. 2013 Jun 10;4:138. doi: 10.3389/fimmu.2013.00138. eCollection 2013. Front Immunol. 2013. PMID: 23772226 Free PMC article. - HIF-1α influences myeloid cell antigen presentation and response to subcutaneous OVA vaccination.
Bhandari T, Olson J, Johnson RS, Nizet V. Bhandari T, et al. J Mol Med (Berl). 2013 Oct;91(10):1199-205. doi: 10.1007/s00109-013-1052-y. Epub 2013 May 19. J Mol Med (Berl). 2013. PMID: 23686259 Free PMC article. - Systemic VHL gene functions and the VHL disease.
Bader HL, Hsu T. Bader HL, et al. FEBS Lett. 2012 Jun 4;586(11):1562-9. doi: 10.1016/j.febslet.2012.04.032. Epub 2012 Apr 25. FEBS Lett. 2012. PMID: 22673568 Free PMC article. Review.
References
- Vogelberg KH, Konig M. Hypoxia of diabetic feet with abnormal arterial blood flow. Clin. Investig. 1993;71:466–470. - PubMed
- Semenza GL. HIF-1 and mechanisms of hypoxia sensing. Curr. Opin. Cell Biol. 2001;13:167–171. - PubMed
- Flamme I, et al. HRF, a putative basic helix-loop-helix-PAS-domain transcription factor is closely related to hypoxia-inducible factor-1α and developmentally expressed in blood vessels. Mech. Dev. 1997;63:51–60. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases