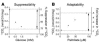Dynamic changes in fat oxidation in human primary myocytes mirror metabolic characteristics of the donor - PubMed (original) (raw)
Dynamic changes in fat oxidation in human primary myocytes mirror metabolic characteristics of the donor
Barbara Ukropcova et al. J Clin Invest. 2005 Jul.
Abstract
Metabolic flexibility of skeletal muscle, that is, the preference for fat oxidation (FOx) during fasting and for carbohydrate oxidation in response to insulin, is decreased during insulin resistance. The aim of this study was to test the hypothesis that the capacity of myotubes to oxidize fat in vitro reflects the donor's metabolic characteristics. Insulin sensitivity (IS) and metabolic flexibility of 16 healthy, young male subjects was determined by euglycemic hyperinsulinemic clamp. Muscle samples were obtained from vastus lateralis, cultured, and differentiated into myotubes. In human myotubes in vitro, we measured suppressibility (glucose suppression of FOx) and adaptability (an increase in FOx in the presence of high palmitate concentration). We termed these dynamic changes in FOx metabolic switching. In vivo, metabolic flexibility was positively correlated with IS and maximal oxygen uptake and inversely correlated with percent body fat. In vitro suppressibility was inversely correlated with IS and metabolic flexibility and positively correlated with body fat and fasting FFA levels. Adaptability was negatively associated with percent body fat and fasting insulin and positively correlated with IS and metabolic flexibility. The interindividual variability in metabolic phenotypes was preserved in human myotubes separated from their neuroendocrine environment, which supports the hypothesis that metabolic switching is an intrinsic property of skeletal muscle.
Figures
Figure 1
Characteristics of in vitro metabolic switching assays. (A) Dose response of FOx (measured by 14CO2 production) to an increasing glucose concentration (suppressibility). (B) Dose response of FOx (measured by 14CO2 production) to an increasing palmitate concentration (adaptability). Arrows indicate a dynamic change in FOx, representing an in vitro correlate to the clinical phenotype. Muscle cells were grown and differentiated into myotubes in 24-well plates. FOx assays were performed after 5 days of differentiation. Myotubes were incubated for 3 hours in serum-free media with increasing concentrations of glucose or palmitate. Assays were performed in duplicate, and data were normalized to protein content. Data are from 1 representative experiment.
Figure 2
In vitro suppressibility is an intrinsic characteristic of muscle cells. The potential of glucose to suppress FOx (CO2) in vitro (% suppression of 14CO2 = [1 – (FOx at 5 mM glucose / FOx at 0 mM glucose)] × 100) is correlated with in vivo metabolic flexibility [as indicated by ΔRQ (clamp), an insulin-stimulated change in RQ, measured by indirect calorimetry during the clamp] (A); in vivo IS represented by glucose disposal rate, measured by clamp (B); percent body fat, measured by dual energy X-ray absorptiometry (C); and in vivo fasting FFA levels (D). Muscle cells from 16 individuals were grown and differentiated into myotubes in 24-well plates. Myotubes were preincubated in glucose- and serum-free medium and incubated for 3 hours with 1 μCi/ml 14C-palmitate, without glucose (to measure maximal FOx) or with 5 mM glucose (to measure suppressed FOx). After incubation, 14CO2 and 14C-intermediate metabolites of FOx were determined. Assays were performed in duplicates and data were normalized to protein content.
Figure 3
In vitro adaptability is an intrinsic characteristic of muscle cells. The capacity of muscle cells to increase FOx (measured by 14CO2 production) in the presence of high palmitate concentration (fold increase in 14CO2 = 14CO2 at 100 μM palmitate / 14CO2 at 0 μM palmitate) in vitro is correlated with flexible clinical phenotype [ΔRQ (clamp)] (A); insulin-sensitive clinical phenotype (IS is represented by the glucose disposal rate, measured by clamp) (B); VO2max in vivo (C); percent body fat, measured by dual energy X-ray absorptiometry (D); and fasting insulin levels on a standard diet (E). Muscle cells from 16 individuals were grown and differentiated into myotubes in 24-well plates. Myotubes were preincubated in glucose- and serum-free medium and incubated for 3 hours with 1 μCi/ml 14C-palmitate, in the presence or absence of 100 μm cold palmitate. After incubation, levels of 14CO2 and 14C-intermediate metabolites of FOx were determined. Assays were performed in duplicate, and data were normalized to protein content. Data were adjusted for basal 14CO2 production (14CO2 production at 1 μCi/ml labeled palmitate and 0 μM cold palmitate).
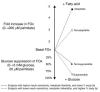
Figure 4
Model of in vitro metabolic switching. Glucose suppressibility: In insulin-free medium, adaptable cells are able to maintain a relatively high rate of FOx in the presence of glucose compared with nonadaptable cells. According to our studies, in vivo insulin responsiveness decreases with increasing in vitro suppressibility, which indicates greater glucose suppression of FOx in insulin resistance and metabolic inflexibility. Metabolic adaptability: Adaptable cells possess a higher capacity to increase FOx when exposed to a high palmitate concentration compared with nonadaptable cells. According to our studies, in vivo insulin responsiveness increases with increasing in vitro adaptability, which indicates a greater capacity to increase FOx in subjects with higher IS.
Comment in
- Skeletal muscle fat oxidation: timing and flexibility are everything.
Kelley DE. Kelley DE. J Clin Invest. 2005 Jul;115(7):1699-702. doi: 10.1172/JCI25758. J Clin Invest. 2005. PMID: 16007246 Free PMC article. Review.
Similar articles
- Effect of adipose tissue on the sexual dimorphism in metabolic flexibility.
Sparks LM, Pasarica M, Sereda O, deJonge L, Thomas S, Loggins H, Xie H, Miles JM, Smith SR. Sparks LM, et al. Metabolism. 2009 Nov;58(11):1564-71. doi: 10.1016/j.metabol.2009.05.008. Metabolism. 2009. PMID: 19595383 - High circulating retinol-binding protein 4 is associated with elevated liver fat but not with total, subcutaneous, visceral, or intramyocellular fat in humans.
Stefan N, Hennige AM, Staiger H, Machann J, Schick F, Schleicher E, Fritsche A, Häring HU. Stefan N, et al. Diabetes Care. 2007 May;30(5):1173-8. doi: 10.2337/dc06-2342. Epub 2007 Jan 26. Diabetes Care. 2007. PMID: 17259477 - Enhanced glucose metabolism is preserved in cultured primary myotubes from obese donors in response to exercise training.
Bourlier V, Saint-Laurent C, Louche K, Badin PM, Thalamas C, de Glisezinski I, Langin D, Sengenes C, Moro C. Bourlier V, et al. J Clin Endocrinol Metab. 2013 Sep;98(9):3739-47. doi: 10.1210/jc.2013-1727. Epub 2013 Jul 24. J Clin Endocrinol Metab. 2013. PMID: 23884778 Clinical Trial. - Type 2 diabetes mellitus and skeletal muscle metabolic function.
Phielix E, Mensink M. Phielix E, et al. Physiol Behav. 2008 May 23;94(2):252-8. doi: 10.1016/j.physbeh.2008.01.020. Epub 2008 Jan 31. Physiol Behav. 2008. PMID: 18342897 Review. - Skeletal muscle fat oxidation: timing and flexibility are everything.
Kelley DE. Kelley DE. J Clin Invest. 2005 Jul;115(7):1699-702. doi: 10.1172/JCI25758. J Clin Invest. 2005. PMID: 16007246 Free PMC article. Review.
Cited by
- Fat burning capacity in a mixed macronutrient meal protocol does not reflect metabolic flexibility in women who are overweight or obese.
Ahern MM, Artegoitia VM, Bosviel R, Newman JW, Keim NL, Krishnan S. Ahern MM, et al. medRxiv [Preprint]. 2024 Aug 30:2024.08.29.24312791. doi: 10.1101/2024.08.29.24312791. medRxiv. 2024. PMID: 39252930 Free PMC article. Preprint. - Aging delays the suppression of lipolysis and fatty acid oxidation in the postprandial period.
Osmond AD, Leija RG, Arevalo JA, Curl CC, Duong JJ, Huie MJ, Masharani U, Brooks GA. Osmond AD, et al. J Appl Physiol (1985). 2024 Nov 1;137(5):1200-1219. doi: 10.1152/japplphysiol.00437.2024. Epub 2024 Sep 5. J Appl Physiol (1985). 2024. PMID: 39236144 - Sedentary time associates detrimentally and physical activity beneficially with metabolic flexibility in adults with metabolic syndrome.
Garthwaite T, Sjöros T, Laine S, Koivumäki M, Vähä-Ypyä H, Verho T, Norha J, Kallio P, Saarenhovi M, Löyttyniemi E, Sievänen H, Houttu N, Laitinen K, Kalliokoski KK, Vasankari T, Knuuti J, Heinonen I. Garthwaite T, et al. Am J Physiol Endocrinol Metab. 2024 Apr 1;326(4):E503-E514. doi: 10.1152/ajpendo.00338.2023. Epub 2024 Feb 28. Am J Physiol Endocrinol Metab. 2024. PMID: 38416072 Free PMC article. - From Obesity-Induced Low-Grade Inflammation to Lipotoxicity and Mitochondrial Dysfunction: Altered Multi-Crosstalk between Adipose Tissue and Metabolically Active Organs.
Cavaliere G, Cimmino F, Trinchese G, Catapano A, Petrella L, D'Angelo M, Lucchin L, Mollica MP. Cavaliere G, et al. Antioxidants (Basel). 2023 May 29;12(6):1172. doi: 10.3390/antiox12061172. Antioxidants (Basel). 2023. PMID: 37371902 Free PMC article. Review. - Association Between Adipose Tissue Characteristics and Metabolic Flexibility in Humans: A Systematic Review.
Glaves A, Díaz-Castro F, Farías J, Ramírez-Romero R, Galgani JE, Fernández-Verdejo R. Glaves A, et al. Front Nutr. 2021 Dec 3;8:744187. doi: 10.3389/fnut.2021.744187. eCollection 2021. Front Nutr. 2021. PMID: 34926544 Free PMC article.
References
- Simoneau JA, Kelley DE. Altered glycolytic and oxidative capacities of skeletal muscle contribute to insulin resistance in NIDDM. J. Appl. Physiol. 1997;83:166–171. - PubMed
- He J, Watkins S, Kelley DE. Skeletal muscle lipid content and oxidative enzyme activity in relation to muscle fiber type in type 2 diabetes and obesity. Diabetes. 2001;50:817–823. - PubMed
- Doucet E, Tremblay A, Simoneau JA, Joanisse DR. Skeletal muscle enzymes as predictors of 24-h energy metabolism in reduced-obese persons. Am. J. Clin. Nutr. 2003;78:430–435. - PubMed
- Kelley DE, Goodpaster B, Wing RR, Simoneau JA. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am. J. Physiol. 1999;277:E1130–E1141. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical