Some assembly required: yeast septins provide the instruction manual - PubMed (original) (raw)
Review
Some assembly required: yeast septins provide the instruction manual
Matthias Versele et al. Trends Cell Biol. 2005 Aug.
Abstract
Septins are a family of conserved proteins that form hetero-oligomeric complexes that assemble into filaments. The filaments can be organized into linear arrays, coils, rings and gauzes. They serve as membrane-associated scaffolds and as barriers to demarcate local compartments, especially for the establishment of the septation site for cytokinesis. Studies in budding and fission yeast have revealed many of the protein-protein interactions that govern the formation of multi-septin complexes. GTP binding and phosphorylation direct the polymerization of filaments that is required for septin-collar assembly in budding yeast, whereas a homolog of anillin instructs timely formation of the ring of septin filaments at the medial cortex in fission yeast. These insights should aid understanding of the organization and function of the diverse septin structures in animal cells.
Figures
Figure 1. The primary structure of a typical septin and organization of septin complexes
(a) The recognizable motifs and domains in the primary structure of a septin. The GTP-binding domain (solid blue) contains all five of the signature motifs (G-boxes) that are found in other members of the super-family of GTPases [81,82]. The predicted coiled-coil sequence in the C-terminal extension (dark gray box) is preceded by a sequence that is predicted to form an α-helix (open box). (b) Proposed organization of multi-septin hetero-oligomers in eukaryotes. Models of the mitotic complexes in budding and fission yeast are adapted from Versele et al. [10] and An et al. [33], respectively. The other models are inferred from the yeast archetypes, based on sequence relatedness, similarities in the primary structure and phylogenetic analysis [5]. Some models are supported by data on individual septin-septin interactions [7,9,45]. In the human complex, Sept9 is shown as the counterpart of Cdc10 (Table 1), based on sequence similarity and phylogenetic-tree analysis [5], the lack of a CTE, and the association between Sept9 and both Sept11 (a Cdc3 ortholog) and Sept7 (a Cdc12 ortholog) [83]. The N-terminal domain of Sept9 is required for its interaction with Sept7 and Sept11, which is similar to the interaction of Cdc10 with Cdc3 and Cdc12. However, there is evidence that the C-terminal portions of Sept7 and of Sept11 are required for their interaction with Sept9 [83], unlike the interactions of Cdc3 and Cdc12 with Cdc10 [10].
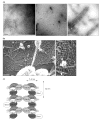
Figure 2. Septin filaments from budding yeast
(a) EM images of negatively-stained preparations of filaments reconstituted from purified, recombinant septin complexes. A multimeric complex of budding yeast septins (Cdc3–Cdc12–Cdc11–Cdc10), purified from_E. coli_ cells in which all four proteins are co-expressed, was dialysed into low-salt buffer. Previously unpublished images (left and middle panel) are courtesy of Sang-Shin Park (this laboratory) and Patricia Grob (laboratory of Eva Nogales, Univ. of California, Berkeley, USA); right panel, reproduced with permission from [10]. Scale bars, 100 nm. (b) EM images in negative-contrast of septin structures at the cell cortex of yeast spheroplasts, prepared by a rapid-freeze, deep-etch technique. Scale bars, 200 nm. Reproduced, with permission, from [13]. (c) Model of the polymerization of septin complexes into paired, linear filaments. End-to-end association of Cdc3 in one Cdc3–Cdc12–Cdc11 complex with Cdc11 in another complex yields filaments with a defined polarity; Cdc10 serves as a bridge to stabilize and pair the strands. A parallel arrangement of filaments is depicted, but an anti-parallel arrangement has not been ruled out by direct experimental evidence. Dimensions are from [8,10].
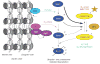
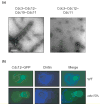
Figure 3
Schematic depiction of septin recruitment and septin-collar formation during the cell cycle of budding yeast. Recruitment of either a patch or cap of septins to the incipient bud site and its rapid conversion into a rimmed disk require the small GTPase, Cdc42. Stabilization of the disk and its transformation into a filamentous collar requires phosphorylation by two proteins kinases, Cla4 and Gin4 (and, perhaps, others), plus binding of GTP to Cdc10 and Cdc12 in the septin complex. At cytokinesis, the collar splits into two separate rings, which are disassembled after cell separation. Confocal fluorescence images (GFP–Cdc12) were kindly provided by Jeroen Dobbelaere (ETH, Zürich, Switzerland).
Similar articles
- Phosphatidylinositol-4,5-bisphosphate promotes budding yeast septin filament assembly and organization.
Bertin A, McMurray MA, Thai L, Garcia G 3rd, Votin V, Grob P, Allyn T, Thorner J, Nogales E. Bertin A, et al. J Mol Biol. 2010 Dec 10;404(4):711-31. doi: 10.1016/j.jmb.2010.10.002. Epub 2010 Oct 15. J Mol Biol. 2010. PMID: 20951708 Free PMC article. - Septin filament compaction into rings requires the anillin Mid2 and contractile ring constriction.
Arbizzani F, Mavrakis M, Hoya M, Ribas JC, Brasselet S, Paoletti A, Rincon SA. Arbizzani F, et al. Cell Rep. 2022 Apr 19;39(3):110722. doi: 10.1016/j.celrep.2022.110722. Cell Rep. 2022. PMID: 35443188 - An anillin homologue, Mid2p, acts during fission yeast cytokinesis to organize the septin ring and promote cell separation.
Tasto JJ, Morrell JL, Gould KL. Tasto JJ, et al. J Cell Biol. 2003 Mar 31;160(7):1093-103. doi: 10.1083/jcb.200211126. J Cell Biol. 2003. PMID: 12668659 Free PMC article. - Septin structure and function in yeast and beyond.
Oh Y, Bi E. Oh Y, et al. Trends Cell Biol. 2011 Mar;21(3):141-8. doi: 10.1016/j.tcb.2010.11.006. Epub 2010 Dec 20. Trends Cell Biol. 2011. PMID: 21177106 Free PMC article. Review. - Reuse, replace, recycle. Specificity in subunit inheritance and assembly of higher-order septin structures during mitotic and meiotic division in budding yeast.
McMurray MA, Thorner J. McMurray MA, et al. Cell Cycle. 2009 Jan 15;8(2):195-203. doi: 10.4161/cc.8.2.7381. Cell Cycle. 2009. PMID: 19164941 Free PMC article. Review.
Cited by
- The Roles of Septins in Regulating Fission Yeast Cytokinesis.
Zheng S, Zheng B, Fu C. Zheng S, et al. J Fungi (Basel). 2024 Jan 30;10(2):115. doi: 10.3390/jof10020115. J Fungi (Basel). 2024. PMID: 38392788 Free PMC article. Review. - Lipid polarity is maintained in absence of tight junctions.
Ikenouchi J, Suzuki M, Umeda K, Ikeda K, Taguchi R, Kobayashi T, Sato SB, Kobayashi T, Stolz DB, Umeda M. Ikenouchi J, et al. J Biol Chem. 2012 Mar 16;287(12):9525-33. doi: 10.1074/jbc.M111.327064. Epub 2012 Jan 31. J Biol Chem. 2012. PMID: 22294698 Free PMC article. - Here come the septins: novel polymers that coordinate intracellular functions and organization.
Spiliotis ET, Nelson WJ. Spiliotis ET, et al. J Cell Sci. 2006 Jan 1;119(Pt 1):4-10. doi: 10.1242/jcs.02746. J Cell Sci. 2006. PMID: 16371649 Free PMC article. Review. - The yeast endocytic protein Epsin 2 functions in a cell-division signaling pathway.
Mukherjee D, Coon BG, Edwards DF 3rd, Hanna CB, Longhi SA, McCaffery JM, Wendland B, Retegui LA, Bi E, Aguilar RC. Mukherjee D, et al. J Cell Sci. 2009 Jul 15;122(Pt 14):2453-63. doi: 10.1242/jcs.041137. Epub 2009 Jun 16. J Cell Sci. 2009. PMID: 19531587 Free PMC article. - Septins of Platyhelminths: identification, phylogeny, expression and localization among developmental stages of Schistosoma mansoni.
Zeraik AE, Rinaldi G, Mann VH, Popratiloff A, Araujo AP, Demarco R, Brindley PJ. Zeraik AE, et al. PLoS Negl Trop Dis. 2013 Dec 19;7(12):e2602. doi: 10.1371/journal.pntd.0002602. eCollection 2013. PLoS Negl Trop Dis. 2013. PMID: 24367716 Free PMC article.
References
- Hartwell L. Genetic control of the cell division cycle in yeast. IV Genes controlling bud emergence and cytokinesis. Exp Cell Res. 1971;69:265–276. - PubMed
- Neufeld TP, Rubin GM. The Drosophila peanut gene is required for cytokinesis and encodes a protein similar to yeast putative bud neck filament proteins. Cell. 1994;77:371–379. - PubMed
- Kinoshita M, et al. Nedd5, a mammalian septin, is a novel cytoskeletal component interacting with actin-based structures. Genes Dev. 1997;11:1535–1547. - PubMed
- Hall PA, et al. Expression profiling the human septin gene family. J Pathol. 2005;206:269–278. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases