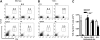T-bet is required for optimal proinflammatory CD4+ T-cell trafficking - PubMed (original) (raw)
T-bet is required for optimal proinflammatory CD4+ T-cell trafficking
Graham M Lord et al. Blood. 2005.
Abstract
Inflammatory responses are controlled by T helper 1 (Th1) lymphocytes. An important function of this polarity is the ability of T cells to traffick appropriately in vivo. This differential trafficking is dependent upon the binding of P-selectin glycoprotein ligand-1 to P- and E-selectin on inflamed endothelium as well as the expression of specific chemokine receptors. Here we show that in the absence of T-box expressed in T cells (T-bet), selective migration of T cells in vivo is completely abrogated and that T-bet regulates the binding of CD4(+) T cells to P-selectin. T-bet is also required for the expression of the chemokine receptor CXCR3. Thus, T-bet controls Th1-cell migration to inflammatory sites, which has fundamental consequences for the control of immunologic disease.
Figures
Figure 1.
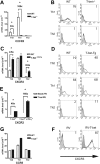
In vivo trafficking of adoptively transferred WT and T-bet–/– antigen-specific T cells activated under Th1-polarizing conditions. (A-B) Flow cytometric analysis of WT (DO11.10) and T-bet–/– (DO11.10 × T-bet–/–) CD4+ T cells. Percentages of cells positive for CD4 and the clonotypic antibody KJ1-26 are indicated in various secondary lymphoid organs and in inflamed peritoneum (ILN indicates inguinal lymph nodes; MLN, mesenteric lymph nodes; and PL, peritoneal lavage). (C) Cell counts of secondary lymphoid organs and peritoneal lavage from BALB/c mice adoptively transferred with DO11.10 (WT) and DO11.10 × T-bet–/– (T-bet–/–) CD4+ T cells (mean ± SEM, *P < .001) activated with OVA peptide and mitomycin C–treated syngeneic splenocytes. □ indicates WT; ▪, T-bet–/–.
Figure 2.
Mechanistic analysis of the selectin binding properties of WT, T-bet–/–, DO11.10, and DO11.10 × T-bet–/– primary CD4+ T cells under conditions of shear flow. (A-B) Interaction of primary CD4+ T cells of different genotypes with immobilized P-selectin (left columns) and E-selectin (right columns) under conditions of laminar shear stress as indicated. (A) Wild-type (WT, □) and T-bet–/– (▪) CD4+ Th1 cells activated by polyclonal stimulation with CD3 and CD28 antibodies. (B) CD4+ T cells generated from DO11.10 and DO11.10 × T-bet–/– TCR-Tg animals and activated in an antigen-specific manner. (C-D) Interaction of WT (□) and T-bet–/– (▪) Th1 and Th2 cells, activated with plate-bound CD3 and CD28 antibodies, with (C) immobilized P-selectin and (D) E-selectin under conditions of laminar shear stress as indicated. All data are expressed as mean ± SEM.
Figure 3.
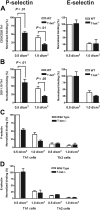
Characterization of a transgenic mouse expressing T-bet under the human CD2 promoter. (A) Western blot of T-bet expression by CD4+ T cells from either WT BALB/c (WT) or T-bet CD2-transgenic (TG) mice (also on the BALB/c background) activated with anti-CD3 and anti-CD28 antibodies under different polarizing conditions. (B-D) Cytokine profiles measured by enzyme-linked immunosorbent assay (ELISA) secreted by CD4+ T cells activated under different polarizing conditions. (B) IFN-γ production during primary stimulation from WT (□) and TG (▪) T cells. (C) IFN-γ production during secondary stimulation from WT and TG T cells. (D) IL-4 production during secondary stimulation from WT and TG T cells. (E-F) Interaction of WT and TG CD4+ T cells with immobilized P-selectin (left columns) and E-selectin (right columns) under conditions of laminar shear stress as indicated. Cells were by stimulated with plate-bound CD3 and CD28 antibodies in the presence of appropriate skewing cytokines. (E) CD4+ Th1-cell interactions. (F) Th2-cell interactions. All data are expressed as mean ± SEM.
Figure 4.
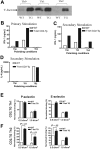
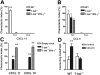
Posttranslational modification of selectin ligands. (A) Flow cytometric surface staining of CD43a (top), CD43c (middle), and PSGL-1 (bottom) on WT and T-bet–/– T cells activated under Th1- or Th2-polarizing conditions (gated on live CD4+ cells; black = isotype, red = WT, and green = T-bet–/–). (B) Real-time PCR analysis of mRNA levels of FucTVII expressed in CD4+ T cells under Th1- and Th2-polarizing conditions (normalized to β-actin). (C) Real-time PCR analysis of mRNA levels of TPST-1 and TPST-2 expressed in CD4+ T cells under Th1-polarizing conditions (normalized to β-actin). □ indicates WT; ▪, T-bet–/–. (D) Autoradiograph of 35S incorporation into PSGL-1, with or without removal of O- and N-linked glycans. (E) Western blot for PSGL-1 in WT and T-bet–/– (knock out [KO]) CD4+ T cells activated under Th1-polarizing conditions (top panel = PSGL-1 dimer; bottom panel = PSGL-1 monomer). (F) Quantification of protein expression of PSGL-1 either with or without removal of O- and N-linked glycans. Results are expressed as mean ± SEM. WB indicates Western blot.
Figure 5.
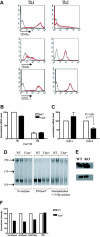
Analysis of chemokine receptor expression in the context of altered T-bet levels in primary CD4+ T cells. Real-time PCR analysis (A,C) and flow cytometric (B,D) surface staining of mRNA levels of CXCR3 in T cells activated under different polarizing conditions (mean ± SEM). (A-B) WT and T-bet–/– CD4+ T cells. (C-D) WT and T-bet CD2-Tg CD4+ T cells. (E) Real-time PCR analysis of CXCR3 expression in primary T-bet–/– and T-bet–/– × IFN-γ–/– CD4+ T cells retrovirally transduced with empty retrovirus (RV) or T-bet RV (mean ± SEM). (F) Flow cytometric surface staining of CXCR3 in retrovirally transduced T cells. (G) mRNA levels of CCR5 in WT and T-bet–/– T cells. All real-time PCR results are normalized to β-actin and are expressed as mean ± SEM (*P < .01). Shaded areas represent isotype staining.
Figure 6.
Responses of primary CD4+ T cells to chemokine ligands in the context of altered T-bet levels. (A-C) Transmigration of CD4+ T cells in response to recombinant chemokine in the lower chamber of a transwell. (A-B) Chemotaxis of WT, T-bet–/–, and T-bet–/– × IFN-γ–/– T cells to recombinant chemokines. (A) Chemotactic response to CXCL11 (I-TAC, 100 nM). □ indicates WT; ▪, T-bet –/–;  , T-bet–/–/IFN-γ–/–.(B) Chemotactic response to CCL4 (MIP-1β, 10 nM). □ indicates WT; ▪, T-bet–/–;
, T-bet–/–/IFN-γ–/–.(B) Chemotactic response to CCL4 (MIP-1β, 10 nM). □ indicates WT; ▪, T-bet–/–;  , T-bet–/–/IFN-γ–/–. (C) Chemotaxis of retrovirally transduced T-bet (or empty vector control) into T-bet–/– and T-bet–/– × IFN-γ–/– T cells to CXCR3 ligands, CXCL11 (100 nM), and CXCL10 (IP-10, 100 nM). □ indicates empty virus; ▪, T-bet–/–;
, T-bet–/–/IFN-γ–/–. (C) Chemotaxis of retrovirally transduced T-bet (or empty vector control) into T-bet–/– and T-bet–/– × IFN-γ–/– T cells to CXCR3 ligands, CXCL11 (100 nM), and CXCL10 (IP-10, 100 nM). □ indicates empty virus; ▪, T-bet–/–;  , T-bet–/–/IFN-γ–/–. (D) Attachment of WT and T-bet–/– T cells to unstimulated endothelial cells under shear flow in the absence or presence of CXCL10 (40 ng/mL) (mean ± SEM). □ indicates control; ▪, CXCL10. **P < .05.
, T-bet–/–/IFN-γ–/–. (D) Attachment of WT and T-bet–/– T cells to unstimulated endothelial cells under shear flow in the absence or presence of CXCL10 (40 ng/mL) (mean ± SEM). □ indicates control; ▪, CXCL10. **P < .05.
Similar articles
- CD43 collaborates with P-selectin glycoprotein ligand-1 to mediate E-selectin-dependent T cell migration into inflamed skin.
Matsumoto M, Shigeta A, Furukawa Y, Tanaka T, Miyasaka M, Hirata T. Matsumoto M, et al. J Immunol. 2007 Feb 15;178(4):2499-506. doi: 10.4049/jimmunol.178.4.2499. J Immunol. 2007. PMID: 17277158 - P-Selectin glycoprotein ligand 1 (PSGL-1) is a physiological ligand for E-selectin in mediating T helper 1 lymphocyte migration.
Hirata T, Merrill-Skoloff G, Aab M, Yang J, Furie BC, Furie B. Hirata T, et al. J Exp Med. 2000 Dec 4;192(11):1669-76. doi: 10.1084/jem.192.11.1669. J Exp Med. 2000. PMID: 11104809 Free PMC article. - Temporal dissection of T-bet functions.
Matsuda JL, George TC, Hagman J, Gapin L. Matsuda JL, et al. J Immunol. 2007 Mar 15;178(6):3457-65. doi: 10.4049/jimmunol.178.6.3457. J Immunol. 2007. PMID: 17339440 - A crucial role for T-bet in selectin ligand expression in T helper 1 (Th1) cells.
Underhill GH, Zisoulis DG, Kolli KP, Ellies LG, Marth JD, Kansas GS. Underhill GH, et al. Blood. 2005 Dec 1;106(12):3867-73. doi: 10.1182/blood-2005-03-0984. Epub 2005 Aug 11. Blood. 2005. PMID: 16099875 Free PMC article. - Regulation of T cell trafficking by the T cell immunoglobulin and mucin domain 1 glycoprotein.
Angiari S, Constantin G. Angiari S, et al. Trends Mol Med. 2014 Dec;20(12):675-84. doi: 10.1016/j.molmed.2014.10.003. Epub 2014 Oct 31. Trends Mol Med. 2014. PMID: 25457618 Review.
Cited by
- T-Bet and Eomes Regulate the Balance between the Effector/Central Memory T Cells versus Memory Stem Like T Cells.
Li G, Yang Q, Zhu Y, Wang HR, Chen X, Zhang X, Lu B. Li G, et al. PLoS One. 2013 Jun 27;8(6):e67401. doi: 10.1371/journal.pone.0067401. Print 2013. PLoS One. 2013. PMID: 23826287 Free PMC article. - Oxygen Sensing by T Cells Establishes an Immunologically Tolerant Metastatic Niche.
Clever D, Roychoudhuri R, Constantinides MG, Askenase MH, Sukumar M, Klebanoff CA, Eil RL, Hickman HD, Yu Z, Pan JH, Palmer DC, Phan AT, Goulding J, Gattinoni L, Goldrath AW, Belkaid Y, Restifo NP. Clever D, et al. Cell. 2016 Aug 25;166(5):1117-1131.e14. doi: 10.1016/j.cell.2016.07.032. Cell. 2016. PMID: 27565342 Free PMC article. - Effects of facial nerve axotomy on Th2- and Th1-associated chemokine expression in the facial motor nucleus of wild-type and presymptomatic mSOD1 mice.
Wainwright DA, Xin J, Mesnard NA, Politis CM, Sanders VM, Jones KJ. Wainwright DA, et al. J Neuroimmunol. 2009 Nov 30;216(1-2):66-75. doi: 10.1016/j.jneuroim.2009.09.009. Epub 2009 Oct 8. J Neuroimmunol. 2009. PMID: 19818514 Free PMC article. - T-bet is essential for the progression of experimental autoimmune encephalomyelitis.
Nath N, Prasad R, Giri S, Singh AK, Singh I. Nath N, et al. Immunology. 2006 Jul;118(3):384-91. doi: 10.1111/j.1365-2567.2006.02385.x. Immunology. 2006. PMID: 16827899 Free PMC article. - Generation of functional murine CD11c+ age-associated B cells in the absence of B cell T-bet expression.
Du SW, Arkatkar T, Jacobs HM, Rawlings DJ, Jackson SW. Du SW, et al. Eur J Immunol. 2019 Jan;49(1):170-178. doi: 10.1002/eji.201847641. Epub 2018 Nov 5. Eur J Immunol. 2019. PMID: 30353919 Free PMC article.
References
- Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383: 787-793. - PubMed
- Szabo SJ, Sullivan BM, Peng SL, Glimcher LH. Molecular mechanisms regulating Th1 immune responses. Ann Rev Immunol. 2003;21: 713-758. - PubMed
- Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman GC, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100: 655-669. - PubMed
- Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002;295: 338-342. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials